b S.R. College of Pharmacy, Department of Pharmaceutical Chemistry & Toxicology, Ananthasagar, Warangal, Telangana 506371, India;
c Programa de Desenvolvimento de Fármacos, Instituto de Ciências Biomédicas, Universidade Federal do Rio de Janeiro, Rio de Janeiro, RJ, Brazil
Metal coordination chemistry,in recent years,has provided a significant contribution to the development of efficient diagnostic and therapeutic agents for biomedical applications [1]. Metal coordination complexes offer biological and chemical diversity that is distinct from that of organic drugs. This diversity arises from not only the choice of the metal itself and its oxidation state,but also from the types and numbers of coordinated ligands and the coordination geometry of the complex [2]. Platinum-based drugs are widely used to treat cancer,but their therapeutic use can be altered by intrinsic or acquired resistance and the occurrence of numerous side effects including neurotoxicity,nephrotoxicity, neuropathy,myelosuppression,thrombocytopenia and neutropenia [3]. Ruthenium,a second row transition metal,continues to attract much attention in the world of scientific research,as it possesses a wide array of applicable properties. Rutheniumcontaining complexes have long been known to be well suited for biological applications,and have long been utilized as replacements to popular platinum based-drugs. In particular,ruthenium complexes have attracted significant attention with two complexes, namely NAMI-A3 and KP1019,advancing through clinical trials [4]. The organometallic Ru(II) arene complexes,with the halfsandwich type structure,have demonstrated their potential increasingly [5]. Their coordination sites can be filled with various ligands,which offer numerous possibilities to modulate biological and pharmacological properties by proper ligand selection [6]. Thiosemicarbazones (TSCs) have attracted considerable attention by chemists and biologists because of their wide range of pharmacological effects,and their metal complexes have shown marked antibacterial,antiviral,antifungal and particularly antitumor activity [7]. The complexes consisted of transition metals and TSC ligands usually possess more potent pharmacological effects than the thiosemicarbazone ligands alone [8]. The biological properties of the TSC ligands can be modified and improved by the linkage to transition metal ions [9]. Ruthenium complexes of diimine ligands such as 2,2'-bipyridine (bpy) and 1,10-phenanthroline (phen) are widely used in bioinorganic chemistry as anticancer agents [10, 11, 12, 13, 14] and a few studies have been carried out on the anticancer activity of ruthenium(II) polypyridyl complexes [15, 16, 17, 18, 19],but many more studies need to be undertaken to develop a novel and interesting group of antitumor transition metal complexes. The design,synthesis and reactivity of novel ruthenium complexes have become the central focus of research in several laboratories,including ours. In this manuscript we report on a study of a family of mixed ligand ruthenium complexes of the type [Ru(phen)2(TSZ)]2+Cl2,[Ru(phen)2(INH)]2+Cl2,[Ru(bpy)2(TSZ)]2+Cl2, [Ru(bpy)2(INH)]2+Cl2,where TSZ is a chelating thiosemicarbazone ligand and INH is isonicotinyl hydrazone ligands derived from the various benzaldehyde derivatives. We report the synthesis and characterization of the complexes and their cytotoxicity toward a various human cancer cell lines,and structure activity relationships.
2. ExperimentalThe solvents AR grades were obtained from Sd Fine Chem., Mumbai,and E. Merck,Mumbai. The reagents (puriss grade) were obtained from Fluka and E. Merck. Hydrated ruthenium trichloride was purchased from Loba Chemie,Mumbai,and used as received. All the melting points were determined in open capillary and are uncorrected. UV-vis spectra were on a Jasco spectrophotometer. FTIR spectra were recorded in KBr powder on a Jasco V410 FTIR spectrometer by diffuse reflectance technique. 1H NMR spectra were measured in CDCl3 and DMSO-d6 on a Bruker Ultraspec 500 MHz/AMX 400 MHz/300 MHz spectrometer. The reported chemical shifts were against that of TMS. Mass spectra were recorded on a JEOL JMS600 spectrum with m-NBA matrix.
Synthetic procedure for the preparation of r-btsz and r-binh: A mixture of substituted benzaldehyde (1 mmol) and thiosemicarbazide/ isoniazid (1 mmol) in 100 mL of ethanol was refluxed for 3 h and left overnight. The crude solid was purified by recrystallization from alcohol to give crystals.
Preparation of cis-[bis (S)dichlororuthenium(II)] cis-[Ru(S)2Cl2] (where S = 2,2'-bipyridine/1,10-phenanthroline): [21].
General procedure for preparing-[Ru(S)2(L)Cl2] (where S = 1,10- phenanthroline/2,2'-bipyridine [21]; where L = 2-OMe-btsz,2-Mebtsz, 4-F-btsz,2,4-dinitro-btsz,2-OMe-binh,2-Me-binh,4-F-binh, 2,4-dinitro-binh.
2-MeO-btsz: Yield 85%. FTIR (KBr,cm-1): 3414-3328 (NH2 and NH),3136 (C-H),2989 (C-H),1609 (N-H),1332 (C=S). λmax nm (MeOH): 226,312 and 395. Mp 189-190 ℃. Anal. Calcd. for C9H11N3OS: C,51.65; H,5.30; N,20.08. Found: C,51.26; H,5.24; N, 20.05. 1H NMR (DMSO-d6): δ 11.02 (s,1H),8.22 (s,1H),7.96 (s,1H), 7.78 (s,1H),7.64 (d,2H,J = 8.4 Hz),6.88 (d,2H,J = 8.4 Hz),3.72 (s, 3H,OCH3). ESI-MS: m/z% 209 (100).
2-CH3-btsz: Yield 82%. FTIR (KBr,cm-1): 3448-3298 (NH2 and NH),3142 (C-H),1614 (N-H),1328 (C=S). lmax nm (MeOH): 242, 326,398. Mp 174-176 ℃. Calcd. for C9H11N3S: C,55.93; H,5.74; N, 21.74. Found C,55.89; H,5.70; N,21.68%. 1H NMR (DMSO-d6): δ 11.28 (s,1H),8.18 (s,1H),8.02 (s,1H),7.94 (s,1H),7.72 (d,2H, J = 8.7 Hz),6.84 (d,2H,J = 8.7 Hz),1.62 (s,3H,CH3). ESI-MS: m/z% 193 (100).
4-F-btsz: Yield 88%. FTIR (KBr,cm-1): 3408-3234 (NH2 and N- H),3085 (C-H),1602 (N-H),1325 (C=S). λmax nm (MeOH): 222, 346.Mp191-192 ℃. Anal. Calcd. for C8H8FN3S: C,48.72; H,4.09; N, 21.30. Found: C,48.56; H,4.04; N,21.18. 1H NMR (DMSO-d6): δ 10.98 (s,1H),8.04(s,1H),7.96 (s,1H),7.82 (s,1H),7.64 (d,2H, J = 8.6 Hz),6.88 (d,2H,J = 8.6 Hz),ESI-MS: m/z% 197 (100).
2,4-Di-NO2-btsz: Yield 72%. FTIR (KBr,cm-1): 3398-3249 (NH2 and N-H),3018 (C-H),1664 (NO2),1610 (N-H),1334 (C=S). λmax nm (MeOH): 216,264 and 386. Mp 183-185 ℃. Anal. Calcd. for C8H7N5O4S: C,35.69; H,2.62; N,26.01. Found: C,35.64; H,2.58; N, 25.96. 1H NMR (DMSO-d6): δ 10.82 (s,1H),8.94 (s,1H),8.04 (s,1H), 7.98 (s,1H),7.62 (d,2H,J = 8.6 Hz),6.88 (1H,d),ESI-MS: m/z% 269 (100).
2-OCH3-binh: Yield 85%,FTIR (KBr,cm-1): 3328(NH),3142 (C-H),1682 (C=O),1608 (N-H). λmax nm (MeOH): 246,322, 394. Mp 257-258 ℃. Anal. Calcd. for C14H13N3O2: C,65.87; H,5.13; N,16.46. Found C,65.77; H,5.10; N,16.42%. 1H NMR (DMSO-d6): δ 10.26 (s,1H),9.02 (s,1H,CH=N),8.03-7.78 (m,4H,Ar-H),7.69 (d, 2H,J = 8.7 Hz),7.56 (d,2H,J = 8.4 Hz),3.78 (s,3H,OCH3). ESI-MS: m/z% 255 (100).
2-CH3-binh: Yield 74%,FTIR (KBr,cm-1): 3375 (NH),3094 (C-H),1680 (C=O),1602 (N-H). λmax nm (MeOH): 224,328, 375. Mp 184-186 ℃. Anal. Calcd. for C14H13N3O: C,70.28; H,5.48; N,17.56. Found C,70.22; H,5.43; N,17.52%. 1H NMR (DMSO-d6): δ 10.06 (s,1H),9.02 (s,1H,CH=N),8.21-7.98 (m,4H,Ar-H),7.83 (dd, 2H),7.48 (d,2H,J = 8.4 Hz),1.58 (s,3H,CH3). ESI-MS: m/z% 239 (100).
4-F-binh: Yield 88%,FTIR (KBr,cm-1): 3402 (NH),3125 (C-H), 1682 (C=O),1615 (N-H). λmax nm (MeOH): 228,306,388. Mp 232-233 ℃. Anal. Calcd. for C13H10N3O2F: C,64.19; H,4.14; N, 17.28. Found C,64.17; H,4.08; N,17.22%. 1H NMR (DMSO-d6): δ 9.94 (s,1H,),8.98 (s,1H,),8.12-7.84 (m,4H),7.72 (d,2H,J = 8.7 Hz), 7.48 (dd,2H,J = 8.4 Hz). ESI-MS: m/z% 243 (100).
2,4-Di-NO2-binh: Yield 92%. FTIR (KBr,cm-1): 3402 (N-H), 3128 (C-H),1681 (C=O),1664 (NO2),1614 (N-H). λmax nm (MeOH): 212,284 and 392. Mp 201-202 ℃. Anal. Calcd. for C13H9N5O5: C,49.53; H,2.88; N,22.22. Found: C,49.48; H,2.82; N, 22.16. 1H NMR (DMSO-d6): δ 10.02 (s,1H),8.84 (s,1H),8.06 (d,2H), 7.86 (s,1H),7.58 (d,2H,J = 8.7 Hz),7.24 (d,1H),7.06 (s,1H) ESIMS: m/z% 315 (100).
Ru(Phen)2(2-OMe-btsz)]2+Cl2 (Ru-1): Yield 48%. FTIR (KBr, cm-1): 3453-3284 (NH2 and N-H),3014 (C-H) 1612 (N-H), 1318 (C=S). λmax nm (MeOH): 215,274,326,398 and 444. Anal. Calcd. for C33H27N7ORuS: C,59.09; H,4.06; N,14.62. Found: C, 59.02; H,4.02; N,14.58. 1H NMR (DMSO-d6): δ 10.04 (s,1H),8.96 (d,1H,J = 4.9 Hz),8.88 (t,2H,J = 5.0 Hz),8.74 (s,1H,),8.52 (d,1H, J = 8.7 Hz),8.44-8.12 (m,3H),8.05-7.98 (m,2H),7.94 (d,1H, J = 8.4 Hz),7.84 (d,1H,J = 8.4 Hz) 7.76-7.68 (m,2H) 7.62 (d,1H, J = 5.0 Hz),7.48-7.45 (m,3H),6.91 (s,br,2H),6.74 (d,2H, J = 8.7 Hz),6.14 (s,1H),3.69 (s,3H,OCH3). MS (ESI) (35 eV) m/z%: 670 (100) for [Ru(phen)2(2-MeO-btsz)].
Ru(bpy)2(2-OMe-btsz)]2+Cl2 (Ru-2): Yield 43%. FTIR (KBr, cm-1): 3426-3318 (NH2 and N-H),3125 (C-H) 1610 (N-H), 1325 (C=S). λmax nm (MeOH): 202,248,298,316,385 and 453. Anal. Calcd. for C29H27N7ORuS: C,55.93; H,4.37; N, 15.75. Found: C,55.78; H,4.29; N,15.68. 1H NMR (DMSO-d6): δ 10.16 (s,1H),9.02 (s,1H),8.94 (s,1H),8.32-8.18 (m,4H),8.03-7.97 (m,4H),7.84-7.62 (dd,2H,J = 8.4 Hz),7.48-7.32 (t,2H,J = 8.6 Hz), 7.28-7.26 (s,1H),7.18-6.98 (m,2H),6.93-6.71 (m,2H),6.60 (s, 2H),6.35-6.12 (m,2H),3.72 (s,3H,OCH3). MS (ESI) (35 eV) m/z%: 622 (100) for [Ru(bpy)2(2-MeO-btsz)].
Ru(Phen)2(2-Me-btsz)]2+Cl2 (Ru-3): Yield 46%,black crystals, FTIR (KBr,cm-1): 3398-3264 (NH2 and N-H),3045 (C-H),1632 (N-H),1328 (C=S). Calcd. for C33H27N7RuS: C,60.54; H,4.16; N, 14.97. Found C,60.38; H,4.12,N,14.88%. 1H NMR (DMSO-d6): δ 9.98 (s,1H),9.48 (s,1H,),8.94 (s,1H),8.83 (d,1H,J = 5.0 Hz),8.74 (s, 1H),8.46-8.21-8.12 (m,3H),8.01 (s,2H),7.94-7.82 (m. 3H),7.69- 7.54 (dd,1H,J = 8.1 Hz),7.42-7.28 (m,2H),7.22 (dd,2H,J = 8.7 Hz), 7.14 (d,2H,J = 8.3 Hz),6.95 (d,1H,J = 8.5 Hz),6.98 (d,2H),6.13 (s, 1H),1.52 (s,3H,CH3). MS (ESI) (35 eV) m/z%: 654 (100) for [Ru(phen)2(2-Me-btsz)].
Ru(bpy)2(2-Me-btsz)]2+Cl2 (Ru-4): Yield 52%,black crystals, FTIR (KBr,cm-1): 3409-3219 (NH2 and N-H),3035 (C-H),1615 (N-H),1327 (C=S). Calcd. for C29H27N7RuS: C,57.41; H,4.49; N, 16.16. Found C,57.38; H,4.32; N,16.12%. 1H NMR (DMSO-d6): δ 10.12 (s,1H),8.94 (s,1H),8.84 (s,1H),8.75 (d,1H,J = 5.6 Hz), 8.68 (d,1H,J = 8.0 Hz),8.42 (d,1H,J = 8.0 Hz),8.02-7.88 (m,4H) 7.76-7.58 (m,4H),7.62-7.58 (dd,2H,J = 8.7 Hz),7.42 (d,1H, J = 5.6 Hz),7.26-7.20 (m,3H),6.97 (d,2H,J = 12.0 Hz),6.22 (s,2H),1.61 (s,3H,-CH3). MS (ESI) (35 eV) m/z%: 606 (100) for [Ru(bpy)2(2-Me-btsz)].
Ru(Phen)2(4-F-btsz)]2+Cl2 (Ru-5): Yield 42%,black crystals,FTIR (KBr,cm-1): 3397-3311 (NH2 and N-H),1611 (N-H),1308 (C=S). λmax nm (MeOH): 211,244,294 340,400 and 485. Anal. Calcd. for C32H24FN7RuS: C,58.35; H,3.67; N,14.88. Found: C,58.26; H,3.64; N,14.82. 1H NMR (DMSO-d6): δ 10.24 (s,1H),9.06 (s,1H),8.84 (dd, 2H,J = 5.0 Hz),8.78 (s,1H),8.56 (s,1H),8.20-8.02 (m,3H),7.88 (s,1H),7.62 (d,1H,J = 8.4 Hz) 7.54-7.48 (m,3H) 7.36 (d,1H), 7.28-7.15 (m,3H),6.96 (m,3H),6.78 (d,2H),6.64 (s,1H,). MS (ESI) (35 eV) m/z%: 658 (100) for [Ru(phen)2(4-F-btsz)].
Ru(bpy)2(4-F-btsz)]2+Cl2 (Ru-6): Yield 38%,black crystals,FTIR (KBr,cm-1): 3396-3318 (NH2 and N-H),1609 (N-H),1307 (C=S). λmax nm (MeOH): 223,265,402 and 455. Anal. Calcd. for C28H24FN7RuS: C,55.07; H,3.46; N,16.06. Found: C,55.02; H, 3.42; N,15.98. 1H NMR (DMSO-d6): δ 9.98 (s,1H),9.02 (s,1H),8.92 (m,2H),8.84 (s,1H),8.62 (s,1H),8.14-8.04 (m,4H),7.96 (dd,2H, J = 8.7 Hz),7.84-7.68 (m,4H) 7.44 (d,1H,J = 8.5 Hz),7.38-7.22 (dd, 2H,J = 8.7 Hz),7.04 (m,2H),6.92 (dd,2H,J = 9.2 Hz),6.88 (s,1H). MS (ESI) (35 eV) m/z%: 610 (100) for [Ru(bpy)2(4-F-btsz)].
Ru(Phen)2(2,4-di-NO2-btsz)]2+Cl2 (Ru-7): Yield 44%,black crystals,IR (KBr,cm-1): 3446-3218 (NH2 and N-H),3041 (C-H), 1662 (NO2),1328 (C=S). Calcd. for C32H23N9O4RuS: C,52.60; H, 3.17; N,17.25. Found C,52.56; H,3.14; N,17.20%. 1H NMR (DMSOd6): δ 10.16 (d,1H,J = 5.2 Hz),9.08 (s,1H),8.98 (d,1H,J = 5.6 Hz), 8.76 (d,1H,J = 8.4 Hz),8.34 (d,1H,J = 8.6 Hz),8.36-8.24 (m,3H), 8.22-8.07 (m,3H),7.95 (d,2H,J = 5.1 Hz),7.86-7.79 (m,2H),7.62- 7.58 (s,1H),7.44-7.40 (m,2H),7.36-7.32 (m,2H),6.93 (s,2H),6.77 (d,2H,J = 15.4 Hz),6.11 (s,1H). MS (ESI) (35 eV) m/z%: 730 (100) for [Ru(phen)2(2,4-di-NO2-btsz)].
Ru(bpy)2(2,4-di-NO2-btsz)]2+Cl2 (Ru-8): Yield 44%,black crystals, IR (KBr,cm-1): 3389-3264 (NH2 and N-H),3115 (C-H),1668 (NO2),1612 (N-H),1332 (C=S). Calcd. for C28H23N9O4RuS: C, 49.26; H,3.40; N,18.47. Found C,49.18; H,3.38; N,18.38%. 1HNMR (DMSO-d6): δ 10.09 (d,1H,J = 5.1 Hz),9.02 (s,1H,),8.87 (d,1H, J = 5.6 Hz),8.64 (d,1H,J = 8.3 Hz),8.46 (d,1H,J = 8.6 Hz),8.37-8.19 (m,4H),8.13-8.07 (m,2H),7.93 (d,2H,J = 5.1 Hz),7.84-7.78 (m, 2H),7.64-7.60 (s,1H),7.46-7.43 (m,2H),7.38-7.32 (m,2H),7.04 (s,2H),6.98 (s,1H). MS (ESI) (35 eV) m/z%: 682 (100) for [Ru(bpy)2(2,4-di-NO2-btsz)].
Ru(Phen)2(2-OMe-binh)]2+Cl2 (Ru-9): Yield 44%,black crystals, IR (KBr,cm-1): 3362 (N-H),3098 (C-H),1676 (C=O). Calcd. for C38H29N7O2Ru: C,63.68; H,4.08; N,13.68. Found C,63.59; H,4.02; N,13.58%. 1H NMR (DMSO-d6): δ 10.24. (d,1H,J = 4.9 Hz),9.09 (s, 1H),8.98 (d,1H,J = 5.3 Hz),8.34-8.22 (m,3H),8.14-7.98 (m,3H), 7.89-7.66 (m,2H),7.62-7.48 (m,3H),7.44-7.32 (dd,2H, J = 8.7 Hz),7.38-7.24 (m,3H),7.22-7.14 (s,1H),7.11-7.02 (m, 2H),6.63 (s,2H),6.36-6.15 (m,2H) 3.64 (s,3H,OMe). MS (ESI) (35 eV) m/z%: 716 (100) for [Ru(phen)2(2-OMe-binh)].
Ru(bpy)2(2-OMe-binh)]2+Cl2 (Ru-10): Yield 48%,black crystals, IR (KBr,cm-1): 3298 (N-H),3066 (C-H),1680 (C=O). Calcd. for C34H29N7O2Ru: C,61.07; H,4.37; N,14.66. Found C,61.02; H,4.32; N,14.58%. 1H NMR (DMSO-d6): δ 10.18 (1H,s),9.14 (s,1H),8.97 (d, 1H,J = 5.4 Hz),8.78 (s,1H),8.56 (d,1H,J = 8.6 Hz),8.44-8.23 (m, 4H),8.21-8.08 (m,3H),7.99 (dd,2H,J = 5.1 Hz),7.86-7.74 (m,4H), 7.62 (s,1H),7.48-7.36 (m,3H),7.28 (m,2H),7.01 (dd,2H, J = 8.4 Hz),3.78 (s,3H,OMe). MS (ESI) (35 eV) m/z%: 668 (100) for [Ru(bpy)2(2-OMe-binh)].
Ru(Phen)2(2-Me-binh)]2+Cl2 (Ru-11): Yield 52%,black crystals, IR (KBr,cm-1): 3302 (N-H),3076 (C-H),1682 (C=O). Calcd. for C38H29N7ORu: C,65.13; H,4.17; N,13.99. Found C,65.10; H,4.12, N,13.86%. 1H NMR (DMSO-d6): δ 10.12-10.03 (d,1H,J = 4.9 Hz), 9.56 (s,1H,),8.99 (s,1H),8.72 (s,1H),8.68 (s,1H),8.54-8.46 (dd, 2H,J = 8.4 Hz),8.22-8.14 (m,3H),8.11-8.04 (m,3H),7.98 (dd, 2H,J = 8.7 Hz),7.96-7.84 (m,3H),7.58-7.36 (dd,2H,J = 8.4 Hz), 7.34-7.28 (m,3H),7.23 (d,2H,J = 9.8 Hz),6.98 (s,1H),1.64 (s,3H, CH3). MS (ESI) (35 eV) m/z%: 700 (100) for [Ru(phen)2(2-Mebinh)].
Ru(bpy)2(2-Me-binh)]2+Cl2 (Ru-12): Yield 44%,black crystals, IR (KBr,cm-1): 3269 (N-H),3008 (C-H),1678 (C=O). Calcd. for C34H29N7ORu: C,62.56; H,4.48; N,15.02. Found C,62.48; H,4.42; N,14.98%. 1H NMR (DMSO-d6): δ 9.76 (s,1H),8.95 (s,1H),8.88 (d, 1H,J = 5.6 Hz),8.74-8.69 (dd,2H,J = 8.7 Hz),8.64 (d,1H, J = 8.2 Hz),8.52 (d,1H,J = 8.4 Hz),8.16-8.02 (m,4H),7.89-7.75 (m,4H),7.68-7.54 (dd,2H,J = 8.7 Hz),7.48 (d,1H,J = 5.6 Hz),7.34- 7.25 (m,3H),7.16-7.04 (m,3H),6.86 (d,2H,J = 12.0 Hz),1.64(s,3H, -CH3). MS (ESI) (35 eV)m/z%: 652 (100) for [Ru(bpy)2(2-Me-binh)].
Ru(Phen)2(4-F-binh)]2+Cl2 (Ru-13): Yield 42%,FTIR (KBr,cm-1): 3397-3311 (N-H),1611 (N-H),1308 (C=S). λmaxnm(MeOH): 211, 244,294 340,400 and 485. Anal. Calcd. for C37H26FN7ORu: C, 63.06; H,3.72; N,13.91. Found: C,63.01; H,3.66; N,13.88. 1H NMR (DMSO-d6): δ 10.16 (s,1H),9.16 (s,1H),8.92 (s,1H),8.88 (s,1H), 8.76 (s,1H),8.34-8.16 (m,3H),8.04 (dd,2H,J = 8.7 Hz),7.98-7.78 (m,3H),7.66-7.52 (s,1H),7.46-7.32 (m,3H),7.24 (s,1H),7.18 (dd, 2H,J = 8.4 Hz),6.98 (d,1H,J = 8.5 Hz),6.84 (d,2H,J = 4.9 Hz) 6.75- 6.72 (t,2H,J = 8.4 Hz),6.52 (s,1H).MS (ESI) (35 eV)m/z%: 704 (100) for [Ru(phen)2(4-F-binh)].
Ru(bpy)2(4-F-binh)]2+Cl2 (Ru-14): Yield 38%,black crystals, FTIR (KBr,cm-1): 3366 (N-H),3126 (C-H),1685 (C=O). λmax nm (MeOH): 223,265,402 and 455. Anal. Calcd. for C33H26FN7ORu: C, 60.36; H,3.99; N,14.93. Found: C,60.28; H,3.92; N,14.88. 1H NMR (DMSO-d6): δ 9.92 (s,1H),8.98 (s,1H),8.86 (m,2H),8.78 (s,1H), 8.55 (s,1H),8.24-8.06 (m,4H),8.01 (dd,2H,J = 8.4 Hz),7.98 (d,2H, J = 8.6 Hz) 7.89-7.66 (m,4H) 7.42 (s,1H),7.35-7.21 (dd,2H, J = 5.0 Hz),7.14 (dd,2H,J = 8.4 Hz),6.94 (t,2H,J = 5.0 Hz),6.72 (s, 1H). MS (ESI) (35 eV) m/z%: 656 (100) for [Ru(bpy)2(4-F-binh)].
Ru(Phen)2(2,4-dinitro-binh)]2+Cl2 (Ru-15): Yield 42%,black crystals,FTIR (KBr,cm-1): 3326 (N-H),3041 (C-H),1681 (C=O), 1662 (NO2). Calcd. for C37H25N9O5Ru: C,57.21; H,3.24; N, 16.23. Found C,57.16; H,3.19; N,16.20%. 1H NMR (DMSO-d6): δ 10.28 (s,1H),9.14 (s,1H),8.98 (d,1H,J = 5.6 Hz),8.84 (d,1H, J = 8.3 Hz),8.54 (d,1H,J = 8.7 Hz),8.45-8.22 (m,3H),8.20-8.08 (m, 3H),7.95 (dd,2H,J = 9.4 Hz),7.86-7.72 (m,3H),7.62 (s,1H),7.48- 7.42 (m,3H),6.93 (s,2H),6.82 (d,2H,J = 15.2 Hz),6.24 (s,1H). MS (ESI) (35 eV) m/z%: 776 (100) for [Ru(phen)2(2,4-di-NO2-binh)].
Ru(bpy)2(2,4-dinitro-binh)]2+Cl2 (Ru-16): Yield 44%,black crystals,IR (KBr,cm-1): 3395 (N-H),3088 (C-H),1675 (C=O). Calcd. for C33H25N9O5Ru: C,54.39; H,3.46; N,17.30. Found C, 54.28; H,3.42; N,17.28%. 1H NMR(DMSO-d6): δ 9.82(s,1H),9.06 (s, 1H),8.92 (s,1H),8.84 (s,1H),8.32-8.20 (m,4H),8.04-7.87 (m,4H), 7.75 (d,2H,J = 5.1 Hz),7.68-7.46 (m,3H),7.25-7.21 (dd,2H, J = 8.4 Hz),7.14-7.02 (m,2H),6.96 (s,2H),6.84 (s,1H),6.58 (s,1H). MS (ESI) (35 eV) m/z%: 728 (100) for [Ru(bpy)2(2,4-di-NO2-btsz)].
3. Results and discussion 3.1. ChemistryThe ligands like r-btsz (r-btsz = substituted benzyl thiosemicarbazones) were prepared by reacting substituted benzaldehydes with thiosemicarbazide in alcohol at 1:1 molar ratio (Scheme 1). r-binh (r-btsz = substituted benzyl isonicotinylhydrazones) were prepared by reacting substituted benzaldehydes with thiosemicarbazide in alcohol at 1:1 molar ratio (Scheme 1). The details of the synthetic strategy adopted for the synthesis of these ruthenium homoleptic compounds was as follows. Ruthenium trichloride was refluxed in DMF in the presence of 1,10-phenanthroline/2,2'- bipyridine and in excess of the stoichiometric amount,which afforded the final product cis-bis(1,10-phenanthroline)dichlororuthenium( II)/cis-bis(2,2'-bipyridine) dichlororuthenium (II) [20]. The third ligand was introduced in alcohol in the presence of nitrogen atmosphere (Scheme 2). The structures of the ligands especially r-binh,and r-btsz were capable of exhibiting bidentate behavior. There were very few cases in which the thiosemicarbazide acts as monodentate ligand binding to the metal center through the sulfur atom [21]. In case of ligands (r-btsz) the chelating mode was via sulfur atom and imine nitrogen by coordination covalent bond. In case of ligands (r-binh) the covalent bond formed between metal ion and oxygen atom and coordinate covalent bond with imine nitrogen.

|
Download:
|
| Scheme 1.Synthesis of ligands r-bitsz and r-binh. | |

|
Download:
|
| Scheme 2.Synthesis of [Ru(S)2(L)]Cl2. | |
The infrared spectra of all the ligands and their ruthenium(II) compounds were recorded in KBr powder by diffuse reflectance technique and are reported in their respective titles by tentative assignments. Vibrational frequencies of the r-btsz ligands shown 3450-3230 cm-1 for NH2 and N-H stretching and from 1330 cm-1 to 1310 cm-1 for C=S stretching. In r-binh ligands showed vibrational frequency from 3420 cm-1 to 3210 cm-1 for N-H stretching and from 1690 cm-1 to 1670 cm-1 for (C=O) stretching. Vibrational frequencies of the ruthenium complexes bearing r-btsz ligands shown 3460-3200 cm-1 for NH2 and N-H stretching and from 1335 cm-1 to 1305 cm-1 for C=S stretching. In ruthenium complexes bearing r-binh ligands showed vibrational frequency from 3400 cm-1 to 3250 cm-1 for N-H stretching and from 1685 cm-1 to 1675 cm-1 for (C=O) stretching.
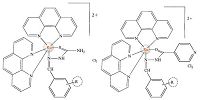
A comparison of IR spectra of ligands r-btsz with ruthenium complexes indicates this was coordinated to the metal center by sulfur atom and imine nitrogen,which was confirmed by the IR spectra. In case of IR spectra of ligands r-binh with ruthenium compound indicates this was coordinated to the metal center by oxygen atom and imine nitrogen. In the complexes such as Ru-1 to Ru-8 the coordination had occurred via sulfur and imine nitrogen but not with terminal amine group,which was confirmed by the spectra,which indicates no change in vibrational frequency of NH2 group between 3400 and 3300 cm-1. A comparison of IR spectra of ligand r-binh with ruthenium complexes indicates this was coordinated to the metal center by oxygen atom and imine nitrogen,which was confirmed by the IR spectra. Coordination of ligands (L = r-binh,r-btsz,) to ruthenium results in compounds such as [Ru(S)2(L)]2+Cl2(Ru1-Ru16) (Fig. 1),respectively. All these compounds do not possess any C2 axes of symmetry.
|
Download:
|
| Fig. 1.Chemical structures of [Ru(phen)2(btsz)]2+ and [Ru(phen)2(binh)]2+. | |
In the 1H NMR spectra of the complexes,Ru-1 there were 24 resonance peaks (δ 10.04-6.14),and 24 well resolved peaks (δ 10.16-6.12) for Ru-2. The mass spectra of the complexes confirmed the suggested formula by their molecular ion peak. ESI mass spectroscopic data clearly suggested that authentification of ruthenium complexes. In all the cases,the loss of chloride ions was detected where S = 2,2'-bipyridine/1,10-phenanthroline and L = rbinh, r-btsz. Thus,based on the above observations,it is tentatively suggested that Ru(II) complexes showed an octahedral geometry.
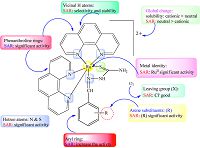
3.2. Biological activityResults are summarized in Tables 1 and 2. The in vitro cytotoxic activity was evaluated for all the synthesized ligands and its ruthenium complexes against human Molt 4/C8,CEM,HL60, BEL7402,and L1210. The relative potencies between ligands and their ruthenium complexes revealed the importance of ruthenium metal using the Molt4/C8,CEM,HL60,BEL 7402 assays and murine L1210 assays. The cytotoxicity data in table revealed that most ruthenium complexes have significant cytotoxic potencies (IC50 figures in the 0.24-2.4 for Molt 4/C8,0.26-1.82 μmol/L for L1210, 0.37-4.6 μmol/L for CEM,0.22-3.5 μmol/L and 0.32-4.2 μmol/L for BEL7402). While for ligands,the IC50 values were in excess (100-228 μmol/L against Molt4/C8,86-198 μmol/L for CEM, 99-248 μmol/L for L1210,66-218 μmol/L against HL60, 99-221 μmol/L for BEL7402). Of the tested ligands and ruthenium complexes Ru(phen)2 (2-(OMe)-btsz)Cl2 showed cytotoxicity against all five cell lines tested in range of 0.34,0.26,0.38,0.22, and 0.48 μmol/L for Molt 4/C8,L1210,CEM,HL60,BEL7402 respectively. Whereas another complex Ru(bpy)2 (2-(OMe)- btsz)Cl2 did show cytotoxicity against cell lines tested 0.62 μmol/L for Molt 4/C8,0.46 for L1210,0.44 for CEM,0.51 for HL60 and 0.32 for BEL7402. Whereas another complex Ru(bpy)2 (2-(OMe)-binh)Cl2 did show cytotoxicity against cell lines tested 1.1 mMfor Molt 4/C8,0.92 for L1210,1.0 for CEM,0.86 for HL60 and 0.94 for BEL7402. Comparison of structure activity relationships (Fig. 2) with ruthenium complexes bearing thiosemicarbazone ligands (Ru-1 to Ru-8) displayed the better cytotoxicity comparison with the ruthenium complexes bearing isonicotinyl hydrazone ligands (Ru-9 to Ru-16). On comparison to ruthenium complexes the ligands displayed the cytotoxicity at higher mmol/L concentration. On comparison with other rutheniumcomplexes (e.g. NAMI-A & KP1019) these reported ruthenium complexes displayed the cytotoxicity at lower μmol/L concentration.
|
|
Table 1 Cytotoxic studies of ligands. |
|
|
Table 2 Cytotoxic studies of ruthenium complexes. |
|
Download:
|
| Fig. 2.Structure activity relationship of [Ru(phen)2(btsz)]2+Cl2. | |
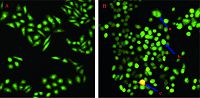
Most of the cytotoxic drugs in current use have been shown to induce apoptosis in susceptible cells. To further address the death pattern,BEL-7402 cells were stained with acridine orange (AO) and ethidium bromide (EB). The AO/EB staining is sensitive to DNA and was used to access changes in nuclear morphology. In the absence of complex Ru-1,the living cells were stained bright green in spots (Fig. 3A). After treatment of BEL-7402 cells with complex (Ru-1) for 48 h,the green apoptotic cells with apoptotic features such as nuclear shrinkage,chromatin condensation,as well as red necrotic cells,were observed (Fig. 3B). Similar results for complexes (Ru-1 to Ru-16) were also observed.
|
Download:
|
| Fig. 3.BEL-7402 cell without treatment (A) and in the presence of Ru complex 2 (B) incubated at 37 ℃ and 5% CO2 for 48 h. Cells in a, b and c are living, apoptotic and necrotic cells, respectively. | |
The compounds prepared in laboratory were evaluated against various cell lines by a literature procedure [23].
4. ConclusionA series of 16 arene Ru(II) complexes (Ru-1 to Ru-16) bearing thiosemicarbazone and ligands were prepared. The cytotoxic activity of the new ruthenium(II) arene compounds has been evaluated in several cell lines (Molt4/C8,L1210,CEM,HL60, BEL7402) in order to establish structure-activity relationships. From the cytotoxic data indicate that ruthenium complexes bearing thiosemicarbzone ligands more active than the ruthenium complexes bearing isonicotinyl hydrazone ligands. It was found that the ruthenium complexes were stronger anticancer activity than the free ligands,it could be due to more efficient uptake of the mononuclear ruthenium complexes. The present study may stimulate the development of novel anticancer molecules,with the increasing competition and reducing development time.
AcknowledgmentsThis work was supported by National Council for Scientific and Technological Development (CNPq),Coordenação de Aperfeic ¸oamento de Pessoal de Nível Superior (CAPES),Oswaldo Cruz Foundation (Fiocruz) and Department of Science and Technology, New Delhi,India [No. SR/WOS-A/LS-562/2011) dated 27/03/2012]. The funders had no role in study design,data collection and analysis,decision to publish,or preparation of the manuscript.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.03.011.
| [1] | M. Patra, G. Gasser, Organometallic compounds: an opportunity for chemical biology? ChemBioChem 13 (2012) 1232-1252. |
| [2] | N.P. Barry, P.J. Sadler, Challenges for metals in medicine: how nanotechnology may help to shape the future, ACS Nano 7 (2013) 5654-5659. |
| [3] | C.M. Clavel, E. Păunescu, P. Nowak-Sliwinska, et al., Discovery of a highly tumorselective organometallic ruthenium(II)-arene complex, J. Med. Chem. 57 (2014) 3546-3558. |
| [4] | C.G. Hartinger, M.A. Jakupec, S. Zorbas-Seifried, et al., KP1019, a new redox-active anticancer agent-preclinical development and results of a clinical phase I study in tumor patients, Chem. Biodivers. 5 (2008) 2140-2155. |
| [5] | I. Romero-Canelón, L. Salassa, P.J. Sadler, The contrasting activity of iodido versus chlorido ruthenium and osmium arene azo-and imino-pyridine anticancer complexes: control of cell selectivity, cross-resistance, p53 dependence, and apoptosis pathway, J. Med. Chem. 56 (2013) 1291-1300. |
| [6] | G.S. Smith, B. Therrien, Targeted and multifunctional arene ruthenium chemotherapeutics, Dalton Trans. 40 (2011) 10793-10800. |
| [7] | D.S. Kalinowski, P. Quach, D.R. Richardson, Thiosemicarbazones: the new wave in cancer treatment, Future Med. Chem. 1 (2009) 1143-1151. |
| [8] | D.R. Richardson, D.S. Kalinowski, V. Richardson, et al., 2-Acetylpyridine thiosemicarbazones are potent iron chelators and antiproliferative agents: redox activity, iron complexation and characterization of their antitumor activity, J. Med. Chem. 52 (2009) 1459-1470. |
| [9] | B.M. Zeglis, V. Divilov, J.S. Lewis, Role of metalation in the topoisomerase IIa inhibition and antiproliferation activity of a series of α-heterocyclic-N4-substituted thiosemicarbazones and their Cu(II) complexes, J. Med. Chem. 54 (2011) 2391-2398. |
| [10] | J. Liu, W.J. Zhen, S. Shi, et al., Synthesis, antitumor activity and structure-activity relationships of a series of Ru(II) complexes, J. Inorg. Biochem. 102 (2008) 193-202. |
| [11] | U. Schatzschneider, J. Niesel, I. Ott, et al., Cellular uptake, cytotoxicity, and metabolic profiling of human cancer cells treated with ruthenium(II) polypyridyl complexes [Ru(bpy)2(N-N)]Cl2 with N-N = bpy, phen, dpq, dppz, and dppn, ChemMedChem 3 (2008) 1104-1109. |
| [12] | Y.J. Liu, C.H. Zeng, H.L. Huang, L.X. He, F.H. Wu, Synthesis, DNA-binding, photocleavage, cytotoxicity and antioxidant activity of ruthenium(II) polypyridyl complexes, Eur. J. Med. Chem. 45 (2010) 564-571. |
| [13] | C.M. Kepert, G.B. Decon, N. Sahely, et al., Synthesis of heteroleptic bis(diimine)-carbonylchlororuthenium(II) complexes from photodecarbonylated precursors, Inorg. Chem. 43 (2004) 2818-2827. |
| [14] | C.P. Tan, J. Liu, H. Li, et al., Differences in structure, physiological stability, electrochemistry, cytotoxicity, DNA and protein binding properties between two Ru(III) complexes, J. Inorg. Biochem. 102 (2008) 347-358. |
| [15] | S.S. Karki, S. Thota, A. Katiyar, et al., Synthesis, characterization and cytotoxic activity of some Ru(II) complexes, Turk. J. Pharm. Sci. 8 (2011) 207-218. |
| [16] | C.P. Tan, S. Lai, S.H. Wu, et al., Nuclear permeable ruthenium(II) b-carboline complexes induce autophagy to antagonize mitochondrial-mediated apoptosis, J. Med. Chem. 53 (2010) 7613-7624. |
| [17] | F. Caruso, E. Monti, J. Matthews, et al., Synthesis, characterization, and antitumor activity of water-soluble (arene)ruthenium(II) derivatives of 1,3-dimethyl-4-acylpyrazolon-5-ato ligands. First example of Ru(arene)(ligand) antitumor species involving simultaneous Ru-N7(guanine) bonding and ligand intercalation to DNA, Inorg. Chem. 53 (2014) 3668-3677. |
| [18] | S. Thota, M. Imran, M. Udugula, et al., Synthesis, spectroscopic characterization and in vitro antitumor activities of some novel mononuclear Ru(II) complexes, Chin. Chem. Lett. 23 (2012) 466-469. |
| [19] | S. Thota, S.S. Karki, K.N. Jayaveera, J. Balzarini, E. De Clercq, Synthesis, characterization, antitumor, and cytotoxic activity of mononuclear Ru(II) complexes, J. Coord. Chem. 63 (2010) 4332-4346. |
| [20] | S. Thota, S.S. Karki, K.N. Jayaveera, J. Balzarini, E. De Clercq, Synthesis, antineoplastic and cytotoxic activities of some mononuclear Ru(II) complexes, J. Enzyme Inhib. Med. Chem. 25 (2010) 513-519. |
| [21] | S.S. Karki, S. Thota, S.Y. Darj, J. Balzarini, E. De Clercq, Synthesis, anticancer, and cytotoxic activities of some mononuclear Ru(II) compounds, Bioorg. Med. Chem. 15 (2007) 6632-6641. |
| [22] | J.A. Hickman, Apoptosis induced by anticancer drugs, Cancer Metastasis Rev. 11 (1992) 121-139. |
| [23] | K. Gangarapu, S. Manda, S. Thota, et al., Microwave assisted synthesis, characterization of some new isatin and thiophene derivatives as cytotoxic and chemopreventive agents, Lett. Drug Des. Discov. 9 (2012) 934-941. |