b National Organization for Drug Control and Research, 6 Hussen Kamal El Deen, Ben-el-sariat, Dokki, Giza 12311, Egypt
Roxatidine (ROX),2-(acetyloxy)-N-[3-[3-(1-piperidinyl methyl) phenoxy] propyl] acetamide is histamine H2-receptor antagonist which also and has anti-ulcerative activity [1]. Orally administered roxatidine acetate is rapidly metabolized to an active metabolite roxatidine,by esterases in the small intestine, liver and plasma [2, 3].
Different chromatographic techniques were applied for the detection and determination of ROX in biological fluids. These techniques include high performance liquid chromatography (HPLC) with UV detector [4],liquid chromatography-mass spectrometry (LC-MS) [5, 6],gas chromatography-mass spectrometry (GC-MS) [7],and capillary gas chromatography with nitrogen selective detection [8]. Also,ROX was determined in pharmaceuticals by spectrophotometry [9] and capillary zone electrophoresis (CZE) [10]. Recently,our work on roxatidine stability by chromatographic methods has been published [11].
Since,some of these methods suffer from either wide spread availability of instrumentation,prohibitive cost or technical difficulty. potentiometric membrane sensors have been more extensively used in pharmaceutical analysis over the last two decades. This technique offers significant promise as an analytical tool in pharmaceutical quality control and coupled with the reliability of the analytical information,leads to attractive approach for the assay of pharmaceutical products. Careful review of the literature found no solid or liquid inner contact ion-selective electrode membranes for determination of ROX.
Design of sensors with improved characteristics of a certain chemical species is quite challenging in chemical research. To this end,we are undertaking this work that involves the design,study and comparison of new electrodes to determine ROX ions in solution. The construction of a polymeric membrane,ion-selective electrode traditionally required a relatively high concentration of the ion of interest in the inner filling solution (IFS); however, experimental evidence suggested that this has a deteriorating influence on the detection limit [12]. Mathison and Bakker [13] showed that an increased concentration of the primary ion in the inner solution leads to its extraction from there together with its counter ions forming ion fluxes from the membrane to the sample. This process changes the ion activity at the phase boundary thus significantly worsening the detection limit. One strategy to counteract this behavior is elimination of the inner solution by using a solid inner contact. In solid-contact SC-ISEs,the sensing membrane is sandwiched between the sample solution and an SCISEs. In recent years,therefore,research has intensified to develop solid contact electrodes with low LODs [14]. To shed light on this concept we have designed two new PVC and graphite-coated ROX sensors and made a comparative study concerning detection limits,concentration ranges,the effect of the internal solution and different plasticizers namely,dibutyl phatalate (DBP) and dibutyl sebacate (DBS),2-nitrophenyl octyl ether (NPOE). These sensors were used for the determination of ROX in bulk powder, pharmaceutical formulation,human plasma and in the presence of its degradation products.
2. Experimental 2.1. InstrumentsA Jenway digital ion analyser model 3330 (Essex,UK) with Orion Ag/AgCl double junction reference electrode (900200 Sure- Flow) was used for potential measurements. A Jenway pH glass electrode no. 924005-BO3-Q11C (Essex,UK) and a Bandelin sonorox magnetic stirrer model Rx 510S (Budapest,Hungary) were used for pH adjustments.
2.2. Chemicals and reagentsRoxatidine acetate hydrochloride reference standard (batch no. R00200-WS-01) was kindly supplied by Sanofi Aventis Deutshland GmbH (Frankfurt,Germany); and certified to contain 99.4% on a dried basis. The commercial Gastralgin® tablets labeled to contain 150 mg of ROX/tab,“BN:N433” were manufactured by De Angeli, AVENTIS PHARMA S.P.A (Milano,Italy).
All chemicals and reagents used throughout this work were of analytical grade (water used was twice-distilled). Polyvinyl chloride (PVC),dibutyl phatalate (DBP) and dibutyl sebacate (DBS) were obtained from Fluka Chemie GmbH (Steinheim, Germany),2-nitrophenyl octyl ether (NPOE) and sodium tetraphenylborate (NaTPB) were purchased from Aldrich (Steinheim, Germany),tetrahydrofuran (THF) BDH (Poole,England),potassium chloride Prolabo (VWR International,West Chester,PA,USA). Britton-Robinson buffer (BRB) (pH 2-12) was prepared by mixing different volumes of 0.04 mol/L acetic acid,0.04 mol/L phosphoric acid,0.04 mol/L boric acid and 0.2 mol/L sodium hydroxide. Fresh human plasma was supplied by (VACSERA,Giza,Egypt) and used within 24 h.
2.3. Procedures 2.3.1. Fabrication of membrane sensorsPreparation of ion-pair association complex: the ion-exchanger complex ROX-tetraphenylborate (ROX-TPB) was prepared by adding a hot solution of 50.0 mL of 0.01 mol/L ROX to 50 mL of 0.01 mol/L sodium tetraphenylborate (TPB). The precipitate that formed was filtered off,washed thoroughly with distilled water, dried at room temperature and ground to fine powders. This ionexchanger complex was used as the active substance for preparing the PVC membrane and coated graphite electrodes of roxatidine acetate.
Preparation of conventional inner contact electrodes,electrode A (sensors 1,2 and 3): [3TD$DIF]In three glass petri dishes (5 cm diameter), 10 mg (1.67%) of the previous association complex (ROX-TPB) was mixed separately,with 400 mg (66.67%) of either o-NPOE (for sensor 1),or DBP (for sensor 2),or DBS (for sensor 3) and 190 mg (31.67%) PVC. Each mixture (totalling 600 mg) was dissolved in 5 mL THF,and the petri dishes were covered with filter paper and left to stand overnight at room temperature to allow solvent evaporation. Master membranes 0.1 mm in thickness were obtained. From each master membrane,a disk (about 8 mm in diameter) was cut using a cork borer and pasted using THF to an interchangeable PVC tip that was clipped into the end of an electrode glass body. The electrodes were then filled with an internal solution of equal volumes of 10-2 mol/L ROX and 10-2 mol/L KCl. Ag/AgCl wire (1 mm diameter) was used as an internal reference electrode. The sensors were conditioned by soaking in 10-2 mol/L aqueous ROX solution for 24 h,and they were stored in the same solution when not in use.
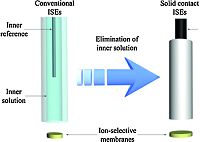
Preparation of the coated-graphite electrodes,electrode B (sensors 4,5 and 6): In three glass petri dishes (5 cm diameter), 10 mg of the previous association complex (ROX-TPB) was mixed separately,with 0.4 mL of either ο-NPOE (for sensor 4),or DBP (for sensor 5),or DBS (for sensor 6) and 0.19 g PVC. Each mixture was dissolved in 5 mL THF,and then the Petri dishes were covered with a filter paper and left to stand for 1 h to allow slow evaporation of the solvent,producing a thick homogeneous master coating PVC solutions. Three rods of spectrographic graphite (5 mmin diameter and 15 mm in length) were inserted separately in a polyethylene sleeve,and about 3 mm of the other end of the protruded rod served as a measuring surface. This end of each rod was washed with acetone,dried in air for 3 h,and dipped rapidly into the previously prepared PVC solutions. The solvent was allowed to evaporate in air after each dipping,and the dipping process was repeated 6-8 times to produce uniform membranes on the surface of each graphite rod. Preconditioning of the coated graphite rods was done by soaking in a 10-2 mol/L ROX solution for 5 h,and stored in the same solution when not in use. Schematic diagrams of (a) liquid and (b) solid inner contact ion-selective electrode membranes are illustrated in Fig. 1.
|
Download:
|
| Fig. 1.Comparison between conventional ion-selective electrodes and solid contacts ion-selective electrodes. | |
The conditioned sensors were calibrated by separately transferring 50 mL aliquots of solutions (10-7 mol/L to 10-2 mol/L) of ROX into a series of 100 mL beakers. The membrane sensors,in conjunction with Ag/AgCl reference electrode,were immersed in the above test solutions and allowed to equilibrate while stirring. The potential was recorded after stabilizing to ±1 mV,and the electromotive force was plotted as a function of the negative logarithm of ROX concentration.
2.3.3. Effect of pHThe effect of pH on the response of the investigated electrodes was studied using 10-3 mol/L and 10-4 mol/L solutions of ROX in BRB with pH ranging from 2 to 10.
2.3.4. Sensors selectivityThe potentiometric selectivity coefficients (KA.B) of the proposed sensors toward different substances were determined by a separate solution method using the following equation [21]:
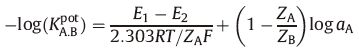
where KA:Bpot is the potentiometric selectivity coefficient,E1 is the potential measured in 10-3 mol/L ROX solution,E2 is the potential measured in 10-3 mol/L interfering solution,ZA and ZB are the charges of ROX and interfering ion,respectively,αA is the activity of the drug and 2.303RT/ZAF represents the slope of the investigated sensors (mV/concentration decade). 2.3.5. Determination of ROX in pharmaceutical preparations
A portion of Gastralgin® tablets powder equivalent to 0.192 g ROX was transferred into 50 mL volumetric flask and filled to the mark with bi-distilled water or BRB solution at pH 7. The concentration of this prepared sample was 1.0 × 10-3 mol/L. The potentiometric measurements were performed using the proposed sensors in conjunction with the Ag/AgCl reference electrode,and the potential readings were compared to the calibration plots.
2.3.6. Determination of ROX in the presence of its alkaline or acid degradation productA degraded sample of ROX was prepared by adding 10 mL NaOH or HCl (1 mol/L) to 10 mL drug solution (10-2 mol/L) and refluxing for 1 h. The resulting solution was tested for complete degradation by TLC using chloroform:methanol:toluene (8:2:2 ratio by volume) as a mobile phase and detecting the peaks at 254 nm. The degraded solution was neutralized,transferred quantitatively into a 100 mL volumetric flask and brought to volume with de-ionized water. Aliquots of the standard drug solution (10-3 mol/L) were mixed with its degraded sample (10-3 mol/L) in different ratios. The emf values of these laboratory-prepared mixtures were recorded and results were compared with the calibration plot.
2.3.7. Determination of ROX in plasmaOne milliliter of each of 10-3 mol/L and 10-4 mol/L standard drug solution were added separately into three 20 mL stoppered shaking tubes each containing 9 mL of plasma and the tubes were shaken for 1 min. The membrane sensors were immersed in conjunction with the reference electrode in these solutions and then washed with water between measurements. The emf produced for each solution was measured by the proposed sensors, and the concentration of ROX was determined from the corresponding regression equation.
3. Results and discussionDesign and development of new electrodes to measure various chemical species,such as ROX,is a prospering area of research. It is rewarding to produce new fabricated electrodes with competitive properties. With these points in mind,we have intimately worked in the design and characterization of these new electrodes: a conventional,liquid inner,contact electrode and a graphite-coated electrode of ROX and then compared their properties in light of these considerations.
3.1. Composition of the electrodesIt is well known that the performance characteristics of ISEs based on ion-exchangers depend,to a large extent,on the nature of these ion-exchangers and their lipophilicities [15],the type of solvent mediator [16] and any additives used [17]. Therefore,the influences of membrane composition and nature of solvent mediator on the potential response of the proposed sensors were tested and the obtained results are presented in this work.
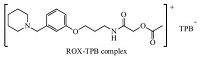
3.1.1. Ion-exchanger selectionIon-exchanger complexes should have appreciable solubility in the membrane matrix and sufficient lipophilicity to prevent leaching from the membrane into the sample solution [18, 19]. The ion-exchanger incorporated in each electrode presented here in Fig. 2 was an ion-association complex of the drug cation with sodium tetraphenyl borate. It was found that NaTPB with its low solubility product and suitable grain size was the optimum cationic exchanger for the formation of 1:1 hydrophobic ion association complex with the studied drug,as proved by elemental analysis,Table 1.
|
Download:
|
| Fig. 2.Suggested structural formula of ion association complex of ROX with NaTPB. | |
|
|
Table 1 Elemental analysis of ROX-TBP complex. |
The solvent mediator,in particular,has a dual function: it acts as a liquefying agent,making the membrane material workable, thus enabling homogenous solubilization and modification of the distribution constant of the ion-exchanger used and sustaining these characteristics on continued use [20]. For a plasticizer to be adequate for use in sensors,it should possess certain properties and characteristics,such as having high lipophilicity,high molecular weight,low tendency for exudation from the membrane matrix,low vapor pressure and high capacity to dissolve the substrate and other additives present in the membrane [21].
To identify a suitable plasticizer for constructing this electrode, we tested three plasticizers with a range of characteristics,namely: DBS,DBP and NPOE. The values of dielectric constants,lipophilicity and molecular weight respectively are in parentheses,for DBS (εr 5.1,PTLC 7.1,m.w. 390),DBP (εr 6.4,PTLC 4.5,m.w. 278),and NPOE (εr 24,PTLC 10.2,m.w. 435). It was determined that the maximum working concentration range and the best Nernstian slopes was observed for electrodes containing the aromatic plasticizer; ο-NPOE (sensors 1 and 4),where the presence of aromatic ring in the plasticizer structure can enhance the solubility of the ion association complex in the electrode matrix [22].
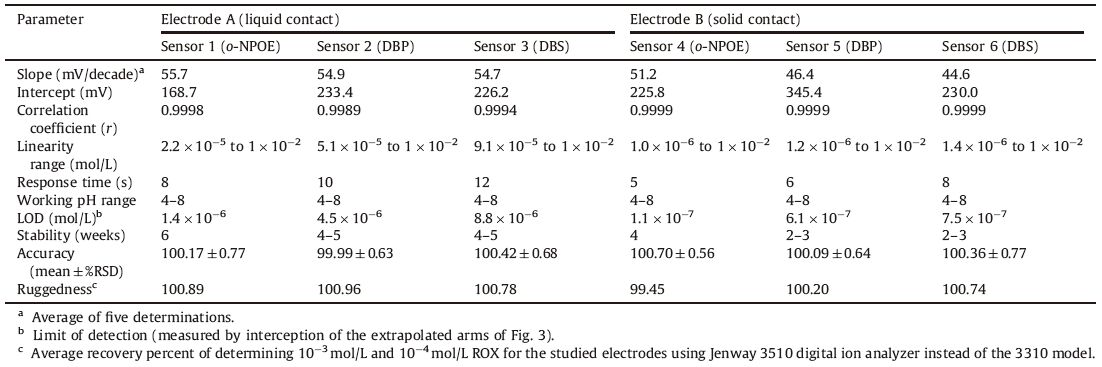
The potentiometric responses of all sensors were examined in the concentration range from 2.0 × 10-8 mol/L to 1.0 × 10-2 mol/L ROX solutions. The calibration plots for these electrodes,represented in Fig. 3,show linearity over the concentration range of 2.2 × 10-5 mol/L to 1.0 × 10-2 mol/L and 1.0 × 10-6 mol/L to 1.0 × 10-2 mol/L and the limits of detection were 1.4 × 10-6 mol/L and 1.1 × 10-7 mol/L for sensors 1 and 4,respectively. The electrochemical performance characteristics of these electrodes were systematically evaluated according to the International Union of Pure and Applied Chemistry (IUPAC) recommendations [23]. Comparison of the slopes,linear ranges and detection limits of the sensors is given in Table 2. The results revealed that the characteristics of solid contact electrode surpass the PVC electrode due to elimination of the internal solution.

|
Download:
|
| Fig. 3.Effect of different plasticizers on the response of electrode A and (liquid) electrode B (solid). | |
|
|
Table 2 Response characteristics of the investigated ROX- selective electrodes. |
For quantitative measurements with ion selective electrodes, studies were carried out to reach the optimum experimental conditions. A pH value within the range 4-8 is found optimum from the point of view of function of the sensors and the chemical form of the test solution of ROX being in the cationic form in acidic media. Figs. 4 and 5 show the potential pH profile for 1.0 × 10-3 mol/L and 1.0 × 10-4 mol/L drug solutions. Above pH 8,the potentials displayed by the sensors sharply decrease due to formation of non-protonated ROX and the OH- ions penetrate the membrane. Below pH 4,the sensors showed nonlinear responses with a slight increase in the potential. This is likely due to the effect of the increase in the hydronium ion concentration on the behavior of the electrodes. Therefore,the pH range from 4 to 8 was assumed to be the working pH range of the six sensors.

|
Download:
|
| Fig. 4.Effect of pH on the response of the proposed liquid contact electrodes. | |

|
Download:
|
| Fig. 5.Effect of pH on the response of the proposed solid contact electrodes. | |
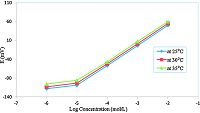
The results suggest that the electrodes exhibit a slight increase in potential with increasing temperature in the range of 25-35 ℃. However,the calibration plots obtained at different temperatures are parallel,and the limits of detection,slopes and response times do not vary significantly with temperature,indicating a reasonable thermal stability of the PVC membranes up to 35 ℃ (Figs. 6 and 7).
|
Download:
|
| Fig. 6.Effect of temperature on the response of the proposed NPOE liquid contact electrode. | |
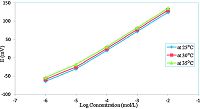
|
Download:
|
| Fig. 7.Effect of temperature on the response of the proposed NPOE solid contact electrode. | |
The dynamic response time is a significant parameter for an ion-selective electrode and was measured according to IUPAC recommendation [23]. The response time may be defined as the time between addition of the analyte to the sample solution and the time when a limiting potential has been reached [23]. In this work,the response time of each electrode was measured by varying the ROX concentration over the range of 5.0 × 10-7 mol/L to 5.0 × 10-2 mol/L. The electrodes reach equilibrium in about 5 s. At lower concentrations,the response time was longer and reached in 8 s and no change was observed up to 5 min. On the other hand, in order to evaluate the reversibility of the proposed electrodes,the electrode potentials of 1.0 × 10-5 mol/L and 1.0 × 10-4 mol/L ROX solutions were measured alternately in the same solution after making the proper treatment. The results indicate that the potentiometric responses of the electrodes are reversible. The life spans of the CWEs,in general,are less than those of the corresponding liquid contact electrodes. This may be attributed to poor mechanical adhesion of the PVC-based sensitive layer to the conductive bed [25].
3.4. Sensors selectivityThe selectivity coefficient is a summary of information concerning interferences on the electrode response in analytical applications and depends on the selectivity of the ion-exchange process at the sensor-test solution interface and the mobility of the respective ions in the matrix of the sensor.
Table 3 shows the potentiometric selectivity coefficients of the proposed sensors in the presence of degradation products,other H2-receptor antagonist drugs,flavoring agents,diluents,excipients (maltose,glucose and lactose) and some other inorganic cations (K+,Na+,and Ca2+) that are usually found in biological fluids. They reflect a very high selectivity of each of these electrodes for the ROX cation over most of the tested species. Overall,the designed electrodes are useful for the intended measurements. The mechanism of selectivity is based mainly on the stereo specificity and electrostatic environment. It is dependent on the extent of fitting between the sites of the lipophilicity of the two competing species in the bathing solution side and the receptor of the ionexchanger [24].
|
|
Table 3 Potentiometric selectivity coefficient (-log Kpot primary ion, interfernt) of the proposed ROX sensors by separate solution method (SSM). |
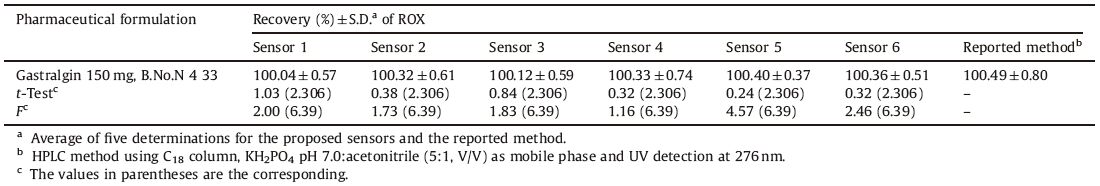
The proposed sensors are applied for the analysis of ROX in pharmaceutical formulations (Gastralgin® tablets) in aqueous and buffered solutions. The results show no significant differences between these two cases. This can be ascribed to the wide working pH range from the acidic side (pH 3) to the basic side (pH 8) of the proposed sensors. The results prove the applicability of the three sensors,as demonstrated by the accurate and precise percentage recovery in Table 4. To examine the validity of the proposed sensors,the obtained results are compared to those of the reported HPLC method [4] and no significant difference for either accuracy or precision was observed. Moreover,the proposed sensors do not require preliminary drug extraction as described in the HPLC method.
|
|
Table 4 Determination of ROX in its pharmaceutical formulation by the suggested potentiometric procedure and reported method. |
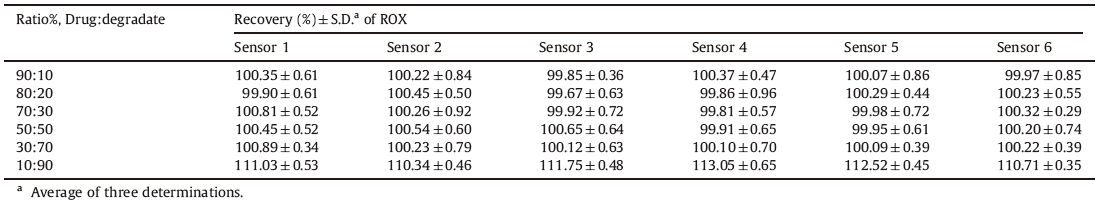
Complete degradation of ROX was induced by refluxing with 1 mol/L NaOH or 1 mol/L HCl for 1 h. The acid and alkaline degradation products were subjected to LC-MS analysis for their identification [11]. Table 5 shows the results obtained upon analysis of synthetic mixtures containing different ratios of intact drug and degraded sample varying from 100:0 to 10:90. The results show that all sensors can be successfully used for selective determination of intact drug in the presence of >70% of its degradation product,but they suffer from high interference when the degradation product concentration reaches about 90%. Thus, they are recommended for use in stability-indicating methods.
|
|
Table 5 Determination of ROX in laboratory prepared mixtures containing different ratios of its degradation products by the proposed sensors. |
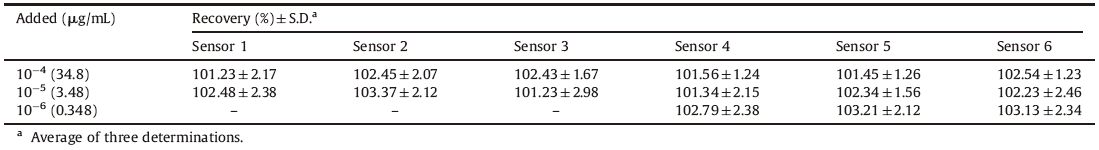
The results obtained for the determination of ROX in spiked human plasma show that a wide concentration range of the drug can be determined by the investigated sensors with high precision and accuracy as presented in Table 6. The response times of the proposed sensors are instant (within 45 s),so the sensors are rapidly transferred back and forth between the plasma samples and the de-ionized,twice-distilled water between measurements to protect the sensing component from adhering to the surface of some matrix components.
|
|
Table 6 Determination of ROX in spiked human plasma by the proposed sensor. |
It is concluded that the proposed sensors can be successfully applied to in vitro studies. It is also clear from the previous results of sensors calibration that the solid contact sensors are the most suitable ones to be used in bioavailability studies as it covers the concentration range 1.0 × 10-6 mol/L to 1 × 10-2 mol/L which is equivalent to the level expected to be found in plasma (Cmax of ROX = 583.13 ± 16.82 ng/mL) (1.5 × 10-6 mol/L) [25].
4. ConclusionsA comparative potentiometric study between two kinds of electrodes (conventional,liquid inner contact and a graphite-coated) was constructed for determination of ROX using different plasticizers. The sensors show favorable performance characteristics with short response times (~8 s),low detection limits of 1.4 × 10-6 mol/L and 1.1 × 10-7 mol/L over the concentration range of 2.2 × 10-5 mol/L to 1 × 10-2 mol/L and 1.0 × 10-6 to 1.0 × 10-2 mol/L,for liquid contact and graphite coated electrodes, respectively. Clearly,the coated graphite sensors show a lower detection limit due to its diminished current flux. The proposed sensors are sufficiently simple and offer advantages of fast response and the elimination of drug pre-treatment or separation steps. Furthermore,the described sensors are selective for the quantitative determination of ROXin pure form,or pharmaceutical formulations,also in the presence of its degradant(s) and in human plasma. Therefore,they can be used for routine analysis of ROX in quality-control laboratories and bioavailability studies.
| [1] | N.J.O. Maryadele, An Encyclopedia of Chemicals, Drug and Biologicals, 14th ed., The Merck Index, Division of Merck and Co. Inc., Merck Research Laboratories, White House Station, NJ, USA, 2006p. 1429. |
| [2] | S. Honma, R. Akutsu, S. Iwamura, Y. Kawabe, K. Tsukamoto, Metabolic fate of 2-acetoxy-N-[3-[m-(1-piperidinylmethyl)phenoxy]propyl] acetamide hydrochloride (TZU-0460), a new H2-receptor antagonist (8) the metabolism in man, Pharmacometrics 30 (1985) 555-563. |
| [3] | W. Rösch, A comparison of roxatidine acetate 150 mg once daily and 75 mg twice daily in gastric ulcer healing, Drugs 35 (1988) 127-133. |
| [4] | C.W. Kuo, W.J. Liaw, P.W. Huang, L.H. Pao, A rapid and sensitive HPLC method for determination of roxatidine in human plasma, J. Food Drug Anal. 16 (2008) 1-5. |
| [5] | B.S. Shin, J.W. Choi, J.P. Balthasar, D.K. Hong, J.J. Kim, S.D. Yoo, Determination of roxatidine in human plasma by liquid chromatography/electrospray mass spectrometry and application to a clinical pharmacokinetic study, Rapid Commun. Mass Spectrom. 21 (2007) 329-335. |
| [6] | J.H. Ryu, S.J. Choi, H.W. Lee, S.K. Choi, K.T. Lee, Quantification of roxadine in human plasma by liquid chromatography electrospray ionization tandem mass spectrometry: application to a bioequivalence study, J. Chromatogr. B 876 (2008) 143-147. |
| [7] | S. Iwamura, K. Shibata, Y. Kawabe, K. Tsukamoto, S. Honma, The metabolism of roxatidine acetate hydrochloride in rat and dog liver homogenates, J. Pharmacobiodyn. 10 (1987) 229-235. |
| [8] | J.L. Burrows, K.W. Jolley, D.J. Sullivan, Determination of roxatidine in human plasma, urine and milk by capillary gas chromatography using nitrogen-selective detection, J. Chromatogr. B 432 (1988) 199-208. |
| [9] | N. Rahman, M. Kashif, Optimized and validated spectrophotometric methods for the determination of roxatidine acetate hydrochloride in drug formulations using 2,3-dichloro-5,6-dicyano-1,4-benzoquinone and p-chloranilic acid, J. Anal. Chem. 60 (2005) 636-643. |
| [10] | J.J.B. Nevado, G.C. Penalvo, R.M.R. Dorado, Evaluation of non-aqueous capillary zone electrophoresis for the determination of histamine H2 receptor antagonists in pharmaceuticals, Anal. Sci. 27 (2011) 427-432. |
| [11] | S.S. Abbas, H.E. Zaazaa, Z.A. EL-Sherif, D.A. Elhadad, B. EL-Zeany, Optimization of stability-indicating chromatographic methods for the determination of roxatidine acetate in the presence of its degradation products, Int. J. Pharm. Pharm. Sci. 6 (2014) 149-157. |
| [12] | A. Radu, A.J. Meir, E. Bakker, Dynamic diffusion model for tracing the real-time potential response of polymeric membrane ion-selective electrodes, Anal. Chem. 76 (2004) 6402-6409. |
| [13] | S. Mathison, E. Bakker, Effect of transmembrane electrolyte diffusion on the detection limit of carrier-based potentiometric ion sensors, Anal. Chem. 70 (1998) 303-309. |
| [14] | E. Bakker, E. Pretsch, Potentiometric sensors for trace-level analysis, TrAC Trends Anal. Chem. 24 (2005) 199-207. |
| [15] | H. Ibrahim, Y.M. Issa, H.M. Abu-Shawish, Improving the detection limits of antispasmodic drugs electrodes by using modified membrane sensors with inner solid contact, J. Pharm. Biomed. Anal. 44 (2007) 8-15. |
| [16] | T. Masadome, J. Yang, T. Imato, Effect of plasticizer on the performance of the surfactant-selective electrode based on a poly(vinyl chloride) membrane with no added ion-exchanger, Microchim. Acta 144 (2004) 217-220. |
| [17] | I. Švancara, K. Vytřas, J. Barek, J. Zima, Carbon paste electrodes in modern electroanalysis, Crit. Rev. Anal. Chem. 31 (2001) 311-345. |
| [18] | H.M. Abu-Shawish, Potentiometric response of modified carbon paste electrode based on mixed ion exchangers, Electroanalysis 20 (2008) 491-497. |
| [19] | V.S. Bhat, V.S. Ijeri, K.A. Srivastava, Coated wire lead(II) selective potentiometric sensor based on 4-tert-butylcalix[6]arene, Sens. Actuators B 99 (2004) 98-105. |
| [20] | H.M. Abu Shawish, A.M. Khedr, K.I. Abed-Almonem, M. Gaber, A comparative study of solid and liquid inner contact benzalkonium chloride ion-selective electrode membranes, Talanta 101 (2012) 211-219. |
| [21] | M.A.A. Pérez, L.P. Marín, J.C. Quintana, M.Y. Pedram, Influence of different plasticizers on the response of chemical sensors based on polymeric membranes for nitrate ion determination, Sens. Actuators B 89 (2003) 262-268. |
| [22] | W. Wroblewski, K. Wojciechowski, A. Dybko, et al., Uranyl salophenes as ionophores for phosphate-selective electrodes, Sens. Actuators B: Chem. 68 (2000) 313-318. |
| [23] | R. IUPAC, Analytical chemistry division, commission on analytical nomenclature, Pure Appl. Chem. 72 (2000) 1851-2082. |
| [24] | N.T. Abdel Ghani, M.S. Rizk, R.M. El-Nashar, Salbutamol plastic membrane electrodes based on individual and mixed ion-exchangers of salbutamolium phosphotungstate and phosphomolybdate, Analyst 125 (2000) 1129-1133. |
| [25] | H.B. Lassman, I. Ho, S.K. Puri, R. Sabo, M.R. Scheffler, The pharmacodynamics and pharmacokinetics of multiple doses of the new H2-receptor antagonist, roxatidine acetate, in healthy men, Drugs 35 (1988) 53-64. |