b Yanbian University Hospital,Yanji 133000,China
cis-Dichlorodiammineplatinum(II) (cisplatin,CDDP),a widely used anticancer drug,shows acute dose-related side effects,such as nephrotoxicity,ototoxicity,neurotoxicity,nausea,vomiting, myelosupression,and the appearance of intrinsic and acquired resistance [1, 2, 3, 4]. Dichloro(1,2-diaminocyclohexane)platinum(II) (DACHPt),a CDDP analog,has shown lower toxicity than CDDP and no cross-resistance with CDDP in many CDDP-resistant cancers [5]. However,it is difficult to prepare injectable formulations because the solubility of DACHPt inwater is much lower than CDDP (0.25 mg/mL for DACHPt vs. 1.2 mg/mL for CDDP) [6]. Recently, many drug delivery systems have been developed to enhance the water solubility of DACHPt. For example,Kataoka et al.,developed PEG-P (Glu) block copolymer micelles containing DACHPt for tumor targeting treatment [7]; DACHPt-loaded micelle exhibited prolonged blood circulation and high tumor accumulation.
It has been reported that DACHPt is sustainably released from the micelles by ligand exchange with the chloride ions in the media [8],discharging approximately 30% of the loaded drugs after 48 h in physiological conditions,while the micelle form is retained [9]. DACHPt-loaded micelles have demonstrated selective tumor accumulation and high antitumor activity in several tumor models [10, 11, 12, 13]. Hyaluronic acid (HA) is a naturally occurring nonsulfated glycosaminoglycan polysaccharide composed of N-acetyl-D-glucosamine and D-glucuronic acid and is a major constituent of the extracellular matrix (ECM) [14, 15]. HA is also known to have pivotal roles in various biological functions,such as stabilizing and organizing the ECM,regulating cell adhesion and motility,andmediating cell proliferation and differentiation [16, 17]. Moreover,HA regulates angiogenesis in many types of tumors,and HA receptors such as CD44 and RHAMM are abundantly present in tumorcells [18, 19]. SinceHAexhibits excellent biocompatibilityand biodegradability,HAand its derivatives have been popularly used as temporal scaffolds for tissue engineering [20] and drug delivery devices for therapeutic agents [21].
To improve the efficacy of cisplatin and control its release, several formulations have been utilized such as nanoparticles [22], liposomes [23],dextrin conjugates [24],and polymeric micelles [25]. The PEGylate polymeric micelle drug carrier system could circulate long-term in the bloodstream and eventually accumulate in the tumor site [26]. The aim of this study is to investigate the feasibility of DACHPt incorporation with PEGylated HA via ion reaction and evaluate its release in vitro and antitumor efficiency in vivo using a lung cancer animal model.
2. Experimental2.1. Materials
Hyaluronic acid (HA,Mw 10-20 kDa) was purchased from Lifecore Biomedical,LLC (Chaska,USA). Methoxy polyethyleneglycol amine (mPEG-NH2; Mw 2000 Da) was synthesized by JenKemTechnology Co. Ltd. (Beijing,China). Dichloro(1,2-diaminocyclohexane) platinum(II) (DACHPt),1-(3-dimethylaminopropyl)-3- ethylcarbodiimide hydro-chloride (EDC),and N-hydroxysuccinimide (NHS) were purchased from Sigma-Aldrich Inc. (Shanghai, China). Silver nitrate (AgNO3) and oxaliplatin were supplied by J&K Chemical Ltd. (Beijing,China). All other reagents were of analytical grade.
2.2. Preparation of mPEG-HAmPEGmodifiedHAwas synthesized in the presence of EDC and NHS,as shown in Fig.1A. Briefly,hyaluronic acid (10 mg) was dissolved in pH 7.4 PBS solution,after which EDC (25.65 mg, 0.13 mmol) and NHS (15.40 mg,0.13 mmol) were added and stirred for 2 h at room temperature. mPEG-NH2 (5.36,10.72, 16.08,21.44 mg) in PBS solution was added to the reaction mixture drop by drop and stirred for another 24 h. The crude product was then purified by dialysis (MWCO 8 kDa) against distilledwater for 48 h with 3 changes a day and characterized by 1H NMR spectroscopy (Varian Mercury-400 MHz spectrometer, Varian Medical Systems Inc.,USA) using D2O as solvents,and further confirmed by Fourier transform infrared spectroscopy (FTIR,Nicolet 5700,Thermo Inc.,USA).
|
Download:
|
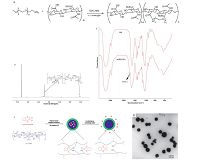
| Fig. 1.The characterization of DACHPt loaded in nanoparticle. (A) The synthesis scheme of mPEG-HA; (B) 600 MHz 1H NMR spectra of mPEG-HA with different PEG substitution degree; (C) IR spectra of mPEG-HA; (D) DACHPt loaded in mPEG-HA nanoparticle was formed and drug replaced by negative charged Cl- which enriched in tumor tissue; (E) Transmission electron microscopy (TEM) images of DACHPt loaded in mPEG-HA nanoparticles. | |
The substitution degree of mPEG was determined by comparing the proton ratios of methylene units in mPEG (-OCH2CH2-: δ 3.72) and methyl groups of HA (-COCH3: δ 2.02[2TD$DIF]) in 1H NMR measurement.
2.3. Preparation of DACHPt-loaded nanoparticlesDACHPt (18.90 mg,0.05 mmol/L) was suspended under ultrasound in distilled water and mixed with AgNO3 (16.99 mg, 0.1 mmol/L) to form the aqueous complex. The solution was kept in darkness at room temperature for 24 h. AgCl precipitates formed after reaction were removed by centrifugation at 3000 rpm for 10 min followed by filtration through a 0.22 μm filter.
mPEG-HA with different substitution degrees was added to the solution of DACHPt aqueous complex ([DACHPt]/[COOH] = 1.0) and stirred for 120 h at room temperature to prepare DACHPtloaded nanoparticles. Unbound DACHPt was removed by ultrafiltration (MWCO: 100 kDa). The size distribution and zeta potential of the DACHPt loaded nanoparticle were evaluated at 25℃ using Malvern Zetasizer Nano ZS90 (Malvern Instruments Ltd.,UK). The Pt content in the nanoparticle was determined by ion coupled plasma-mass spectrometry (ICP-MS,Agilent 7700s,USA). The drug contents and loading efficiency were calculated as follows: [5TD$DIF]Drug content equation,the amount of DACHPt from the nanoparticles divided weight of nanoparticles; the drug efficiency equation,the amount of DACHPt from the nanoparticles divided by the feeding amount of DACHPt.
2.4. In vitro drug release testThe in vitro release rate of the DACHPt from mPEG-HA-DACHPt conjugate with different PEG substitution degrees was determined in saline (pH 7.4,37℃). 1 mg/mL mPEG-HA-DACHPt solution was sealed in a dialysis bag (MWCO 8000,Pierce) and placed in a stirred bath of 40 mL PBS (140 mmol/L,pH 7.4,37℃). 0.2 mL samples were taken from the dialysis bags at predetermined time points and the remaining Pt concentration was determined by ICP-MS. All experiments were conducted in triplicate.
2.5. Cytotoxicity study2.5.1. Cell line and cell culture
Human non-small-cell lung carcinoma A549 cells were provided by the Department of Pathology in Institute of Medicinal Biotechnology in Peking Union Medical College. A549 cells were cultured with Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% (v/v) fetal bovine serum and 0.8 μg/mL puromycin at 37℃ in humidified atmosphere containing 5% CO2. Cells in logarithmic growth phase were used to conduct all cell experiments in this study.
2.5.2. In vitro cytotoxicityThe in vitro cytotoxicity of the mPEG-HA-DACHPt nanoparticle was evaluated using the MTT assay with A549 cells. Briefly,A549 cells (8 × 103 cells/well)were seeded in 96-well plates and cultured for 24 h for adherence. After 24 h,the cells were treated with PBS (control),different concentration of HA,mPEG-HA polymer and different concentration of DACHPt loaded nanoparticles in RPMI 1640 media for 48 h. Thereafter,20 μLMTT solution (5 mg/mL) was added and incubated with cells at 37℃ for 4 h. After the removal of the unreacted MTT dye by aspiration,180 mL of DMSO was added into each well to dissolve the produced formazan crystals,and the plate was gently shaken for 10 min. Finally,the optical density (OD) was measured at 490 nm using Synergy H1m Monochromator- Based Multi-Mode Microplate Reader (BioTek.,USA). The cell viability was calculated according to the below formula:
Cell viability(%)=[(ODsample - ODblank)/(ODcontrol - ODblank)]× 100%
2.5.3. Animal study
The animal experiment was ethically approved by Laboratory Animal Ethics Committee in the Institute of Materia Medica in Peking Union Medical College. All experimental procedures were performed in conformity with institutional guidelines and protocols for the care and use of laboratory animals. Firstly,1 × 105 A549 cells were inoculated into the right rare flank of the nude mice of 6-8 weeks old male BALB/c mice to establish a malignant tumor model. Tumor volumes were monitored with a dial caliper during tumor progression. When tumor volume reached to about 120 mm3 (on the 13th day after inoculation),mice were randomly divided into saline,mPEG-HA-DACHPt (2% substitution degree) and HA-DACHPt nanoparticle groups (n = 5). In each group,mice were intravenously injected with mPEG-HA-DACHPt or HADACHPt nanoparticles at a dose of 4 mg DACHPt/kg body weight on alternate days for a total of 4 times.
3. Results and discussion3.1. Characterization of nanoparticles
mPEG-HA based novel polymeric nanoparticles entrapping DACHPt were prepared through the conjugation between DACHPt and mPEG-HA in distilled water after converting DACHPt to its aqueous complex by pretreatment with AgNO3 to increase water solubility. The structure of the mPEG-HA was confirmed by 1H NMR spectroscopy (Fig.1B) and the substitution degree of mPEG was determined by comparing the proton ratios of methylene units in mPEG (-OCH2CH2-: δ 3.72) and methyl groups of HA (-COCH3: δ 2.02) in the 1H NMR spectrum. IR data (IR Prestige-21 FTIR spectrometer,Shimadzu,Japan): 1736 cm-1 for C-O stretching vibration mode of OHC-PEG-CHO; 1728 cm-1 for C-O stretching vibration mode of Gal-PEG-CHO,1060 cm-1 for C-N stretching vibrations (Fig.1C). The polymeric nanoparticle was formed in the water solution as shown in Fig.1D. Hydrophobic DACHPt is attributing to core phase and the hydrophilic PEG is distributed on the surface of the particles. mPEG-HA nanoparticle was formed and their particle size was distributed around 60-95 nm and the average size was 86 nm with sphere shape determined by TEM. The drug content and the loading efficiency decreased with increasing the PEG substitution degree as shown Table1. However, the particle size decreased when the substitution degree increasedfrom 0 to 5%. It is suggested that the micelle formed more stable nanoparticles when PEG was conjugated with HA.
|
|
Table 1 Characterization of DACHPt loaded in mPEG-HA with the substitution degree 1–5% and DACHPt loaded in HA nanoparticles. |
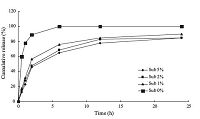
The release of the DACHPt from nanoparticles in saline (pH 7.4) was evaluated by using a dialysis method [25]. The release profile is shown in Fig.2. Release of DACHPt in mPEG-HA nanoparticle was in a sustained manner. DACHPt release from the nanoparticles showed two phases,that is an initial burst release phase until 4 h followed by a continuous release phase until 24 h. The initial release may be caused by DACHPt-mPEG-HA bound at the surface of the nanoparticles,followed by the predicted controlled-release. The DACHPt release slightly decreased with increasing substitution degree of mPEG-HA. It is suggested that the PEG distributed on the surface of the nanoparticle could block ion exchange with core phase drugs,retarding drug release.
|
Download:
|
| Fig. 2.In vitro release profile of DACHPt loaded in mPEG-HA and HA nanoparticles in saline (pH 7.4). | |
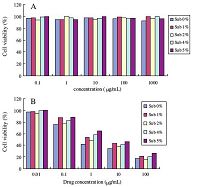
Safety is one of the most important factors to be considered for chemotherapy drug carriers,especially for clinical application. It has been demonstrated that HA was biodegradable and safe for application in vivo. However,mPEG-HAs with different PEG substitution degrees used in this study have never been evaluated for their toxicity. To confirm whether the carrier has toxicity,MTT assays were performed to evaluate the cytotoxicity of mPEG-HA and DACHPt loaded nanoparticles using A549 cell lines. The test concentrations of mPEG-HA ranged from 0.1 μg/mL to 1000 μg/ mL. Cells without treatment of polymer were used as control with a cell viability of 100%. The results of cytotoxicity assay were shown in Fig.3A. With the increment of mPEG-HA’s concentration,it was found that there was no significant reduction of cell viability. Moreover,cell viabilities at different concentrations all exceeded 95% (Fig.3A). Based on these data,it was concluded that mPEG-HA with the substitution degree range from 0% to 5% was a non-toxic and biocompatible carrier. On the other hand,the cytotoxicity of DACHPt loaded nanoparticles with the concentration range 0.01- 100 μg/mL against the A549 cell line showed a lower value with increasing drug concentration (Fig.3B). A high therapeutic index is expected via elevation in the cytotoxicity after sustained release of the drug from nanoparticles in vivo.
|
Download:
|
| Fig. 3.Cell viability of mPEG-HA without DACHPt (A) and with DACHPt in different concentration with different substitution degrees in an A549 cell line (B). | |
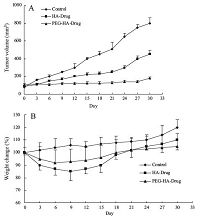
DACHPt loaded in mPEG-HA (2% substitution degree) and HA nanoparticles were employed to inhibit the tumor growth in vivo. Firstly,A459 cells were inoculated into male BALB/c mice to establish a tumor animal model. When tumor volume reached about 120 mm3 (on the 13th day after inoculation),drug loaded nanoparticles were administrated to tumor-bearing mice for antitumor therapy. The results of tumor volume showed that the tumors of mice in themPEG-HA group grew very slowly (Fig.4A), which confirmed that DACHPt loaded mPEG-HA nanoparticles had a strong inhibitory effect on tumor growth in vivo. The body weight change curve shows that the mice weight loss was lessthan 5% by treating with both DACHPt loaded inmPEG-HA and HA nanoparticles as shown in Fig.4B. The treated mice started recovering their body weights soon after drugs were stopped. So, it was concluded that DACHPt loaded in mPEG-HA nanoparticle was able to deliver the DACHPt to effectively inhibit the tumor growth in vivo.
|
Download:
|
| Fig. 4.Effects of the DACHPt loaded in mPEG-HA and HA nanoparticles on the growth of A549 lung carcinoma s.c. transplanted to nu/nu mice (male, n = 5) (A). Body weight changes of A549 tumor bearing nu/nu mice treated with saline, DACHPt loaded in mPEG-HA and HA nanoparticles (B). Each formulation was administered four times at three-day intervals. | |
In summary,we have directly grafted mPEG to hyaluronic acid (HA) molecules to synthesize mPEG-HA with different substitution degrees which have very low toxicity and good biocompatibility. The results suggested that mPEG-HA could load DACHPt to form stable nanoparticles and promote sustained release in vitro. The cytotoxicity of DACHPt loaded in nanoparticle was dose-dependent to lung cancer A459 cell line. mPEG-HA could serve as a highly efficient carrier for DACHPt delivery,especially for anti-tumor therapy.
Acknowledgments
This study was supported by research grants from the National Natural Science Foundation of China (No. 81373342),Beijing Natural Science Foundation (Nos. 2141004,7142114).
| [1] | S. Carrick, D. Ghersi, N. Wilcken, J. Simes, Platinum containing regimens for metastatic breast cancer, Cochrane Database Syst. Rev. (2004) CD003374, http://dx.doi.org/10.1002/14651858.CD003374.pub3. |
| [2] | G. Chu, Cellular-responses to cisplatin—the roles of DNA-binding proteins and DNA-repair, J. Biol. Chem. 269 (1994) 787-790. |
| [3] | D.C. Ihde, J.L. Mulshine, B.S. Kramer, et al., Prospective randomized comparison of high-dose and standard-dose etoposide and cisplatin chemotherapy in patients with extensive-stage small-cell lung-cancer, J. Clin. Oncol. 12 (1994) 2022-2034. |
| [4] | R.P. Perng, Y.M. Chen, M. Lu, et al., Gemcitabine versus the combination of cisplatin and etoposide in patients with inoperable non-small-cell lung cancer in a phase II randomized study, J. Clin. Oncol. 15 (1997) 2097-2102. |
| [5] | B. Desoize, C. Madoulet, Particular aspects of platinum compounds used at present in cancer treatment, Crit. Rev. Oncol. Hematol. 42 (2002) 317-325. |
| [6] | Y. Kidani, K. Inagaki, M. Iigo, A. Hoshi, K. Kuretani, Antitumor activity of 1,2-diaminocyclohexane-platinum complexes against sarcoma-180 ascites form, J. Med. Chem. 21 (1978) 1315-1318. |
| [7] | H. Cabral, N. Nishiyama, S. Okazaki, H. Koyama, K. Kataoka, Preparation and biological properties of dichloro(1,2-diaminocyclo-hexane) platinum (II)(DACHPt)-loaded polymeric micelles, J. Control. Release 101 (2005) 223-232. |
| [8] | H. Cabral, N. Nishiyama, K. Kataoka, Optimization of (1,2-diamino-cyclohexane)-platinum(II)-loaded polymeric micelles directed to improved tumor targeting and enhanced antitumor activity, J. Control. Release 121 (2007) 146-155. |
| [9] | M. Murakami, H. Cabral, Y. Matsumoto, et al., Improving drug potency and efficacy by nanocarrier-mediated subcellular targeting, Sci. Transl. Med. 3 (2011) 64ra2. |
| [10] | H. Cabral, Y. Matsumoto, K. Mizuno, et al., Accumulation of sub-100 nmpolymeric micelles in poorly permeable tumours depends on size, Nat. Nanotechnol. 6 (2011) 815-823. |
| [11] | M. Rafi, H. Cabral, M. Kano, et al., Polymeric micelles incorporating (1,2-diaminocyclohexane) platinum(II) suppress the growth of orthotopic scirrhous gastric tumors and their lymph node metastasis, J. Control. Release 159 (2012) 189-196. |
| [12] | H. Cabral, M. Murakami, H. Hojo, et al., Targeted therapy of spontaneous murine pancreatic tumors by polymeric micelles prolongs survival and prevents peritoneal metastasis, Proc. Natl. Acad. Sci. U. S. A. 110 (2013) 11397-11402. |
| [13] | S. Deshayes, H. Cabral, T. Ishii, et al., Phenylboronic acid-installed polymeric micelles for targeting sialylated epitopes in solid tumors, J. Am. Chem. Soc. 135 (2013) 15501-15507. |
| [14] | J.R. Fraser, T.C. Laurent, Turnover and metabolism of hyaluronan, Ciba Found. Symp. 143 (1989) 41-53 (discussion 53-59, 281-285). |
| [15] | C.B. Knudson, W. Knudson, Hyaluronan-binding proteins in development, tissue homeostasis, and disease, FASEB J. 7 (1993) 1233-1241. |
| [16] | J. Entwistle, C.L. Hall, E.A. Turley, HA receptors: regulators of signaling to the cytoskeleton, J. Cell. Biochem. 61 (1996) 569-577. |
| [17] | T.C. Laurent, Biochemistry of hyaluronan, Acta Otolaryngol. 442 (1987) 7-24. |
| [18] | K. Akima, H. Ito, Y. Iwata, et al., Evaluation of antitumor activities of hyaluronate binding antitumor drugs: synthesis, characterization and antitumor activity, J. Drug Target. 4 (1996) 1-9. |
| [19] | Q. Hua, C.B. Knudson, W.J. Knudson, Internalization of hyaluronan by chondrocytes occurs via receptor mediated endocytosis, J. Cell Sci. 106 (1993) 365-375. |
| [20] | V. Mironov, G.D. Prestwich, Fabrication of tubular tissue constructs by centrifugal casting of cells suspended in an in situ crosslinkable hyaluronan-gelatin hydrogel, Biomaterials 26 (2005) 7628-7635. |
| [21] | M. Kurisawa, H. Uyama, Injectable biodegradable hydrogels composed of hyaluronic acid-tyramine conjugates for drug delivery and tissue engineering, Chem. Commun. 34 (2005) 4312-4314. |
| [22] | K. Avgoustakis, A. Beletsi, Z. Panagi, et al., PLGA-mPEG nanoparticles of cisplatin: in vitro nanoparticle degradation, in vivo drug release and in vivo drug residence in blood properties, J. Control. Release 79 (2001) 123-135. |
| [23] | M.S. Newman, G.T. Colbern, P.K. Working, C. Engbers, M.A. Amantea, Comparative pharmacokinetics, tissue distribution, and therapeutic effectiveness of cisplatin encapsulated in long circulating, pegylated liposomes (SPI-077) in tumor-bearing mice, Cancer Chemother. Pharmacol. 43 (1999) 1-7. |
| [24] | Y. Ohya, T. Masunaga, T. Baba, T. Ouchi, Synthesis and cytotoxic activity of dextran carrying cis-dichloro (cyclohexanetrans-l-1,2-diamine) platinum(II) complex, J. Biomater. Sci. Polym. Ed. 7 (1996) 1085-1096. |
| [25] | N. Nishiyama, K. Kataoka, Preparation and characterization of size-controlled polymeric micelle containing cis-dichlorodiamine-platinium(II) in the core, J. Control. Release 74 (2001) 83-94. |
| [26] | N. Nishiyama, S. Okazaki, H. Cabral, et al., Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice, Cancer Res. 63 (2003) 8977-8983. |





