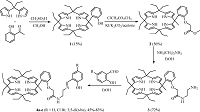
As a representative class of neutral macrocycle receptors, calixpyrrole has become one of the most promising macrocyclic molecules currently being explored for the purpose of anion binding [1, 2, 3, 4]. Efforts to improve the anion affinity of calixpyrrole and to enhance its selectivity have led to a variety of chemical modifications of calixpyrrole skeleton and substituents. Thus,a vast range of functionalized calixpyrroles derivatives equipped with various chromogenic,fluorogenic,or redoxactive units as well as others are successfully developed [5, 6, 7, 8, 9, 10, 11, 12, 13, 14]. On the other hand, calixpyrrole core are widely used as tetraanionic N4-ligands for metal coordination chemistry [15, 16, 17, 18, 19, 20, 21]. A special class of macrocyclic Schiff-base calix[4]pyrroles,in which Schiff-base unit is incorporated in the macrocyclic ring,have showed good complexing ability for transition metal ion and rare earth metal ions [22- 25]. However,there are few reports about introduction of functionalized coordination moieties onto calix[4]pyrrole platform and transition metal complexes [26, 27]. Against this background and in continuation of our aim to investigate the metallic complexes of the functionalized macrocyclic polydentated ligands [28, 29, 30],recently we found the efficient synthetic procedure for the 15a,20a-di(4-hydroxylphenyl)calix[4]pyrroles for the first time by the acidic catalyzed cyclization reaction between 5,50- dialkyldipyrromethanes and p-hydroxyacetophenone [31, 32]. We also synthesized a series of calix[4]pyrrole 10a,20a-disubstituted Schiff bases and urea derivatives by using corresponding 10a,20adi( 4-nitrophenyl)calix[4]pyrroles as starting materials [33]. Herein we wish to report the synthesis of calix[4]pyrrole mono-Schiff bases and their transition metal complexes.
2. Experimental2.1. Synthesis of 5-methyl-10,10,15,15,20,20-hexaethyl-5-(2- hydroxylphenyl)calix[4]pyrrole (1)
5,5'-Diethyldipyrromethane (20 mmol,4.04 g) and o-hydroxyacetophenone (20 mmol,2.72 g) were dissolved in methanol (40.0 mL). The solution was stirred under ice-bath. Then methanesulfonic acid (20 mmol,1.92 g) was added. The solution was stirred at room temperature for 12 h. The resulting precipitate was collected by filtration and washed with cool ethanol,which was further titrated with acetone to give the white solid. Then,the crude product was recrystallized from a mixture of methylene dichloride and ethanol (v/v = 6:1) to give the di(2-hydroxyphenyl) calix[4]pyrrole. The mother liquid was concentrated withrotator evaporation and recrystallized with ethanol to give the pure product 1,white solid,15%,mp 244-246 ℃; 1H NMR (600 MHz,DMSO-d6):δ 9.44 (brs,2H,pyrrole-NH),8.89 (brs,2H, pyrrole-NH),7.39 (s,1H,OH),7.04 (t,1H,J = 7.2 Hz,ArH),6.69 (d, 1H,J = 7.8 Hz,ArH),6.65 (t,1H,J = 7.2 Hz,ArH),6.56 (d,1H, J = 7.8 Hz,ArH),5.79 (s,2H,β-pyrrole-H),5.78 (s,2H,β-pyrrole-H), 5.76 (s,2H,β-pyrrole-H),5.71 (s,2H,β-pyrrole-H),2.10-2.05 (m, 2H,CH2),1.95-1.92 (m,4H,CH2),1.83 (s,3H,CH3),1.81-1.75 (m, 4H,CH2),1.71-1.67 (m,2H,CH2),0.64 (t,12H,J = 6.6 Hz,CH3),0.57 (t,3H,J = 7.2 Hz,CH3),0.44 (t,3H,J = 7.2 Hz,CH3); 13C NMR (150 MHz,CDCl3):δ153.7,135.9,135.7,133.9,133.4,128.3,120.9, 118.7,105.9,105.5,104.6,43.1,42.9,42.7,28.9,28.6,28.3,28.0, 25.5,8.1,8.0,7.8; IR (KBr,cm-1)ν: 3901,3434,2958,1574,1460, 1333,1282,1198,1106,1040,929,842,763,701; ESI-HRMS Calcd. for C39H50N4NaO ([M+Na]+) 613.3882,found 613.3877.
2.2. Synthesis of 5-methyl-10,10,15,15,20,20-hexaethyl-5-(2- methoxycarbonylmethoxyphenyl)calix[4]pyrrole (2)A suspension of above prepared compound 1 (1.0 mmol,0.64 g), methyl a-chloroacetate (5.0 mmol,0.54 g),potassium carbonate (30 mmol,4.14 g) and potassium iodide (0.10 g) in dry acetone (30.0 mL) was stirred under reflux for about 8 h. After cooling to room temperature,the solution was filtrated out and evaporated under reduced pressure to give the oil residue,which was titrated with ethanol to give the pure product,white solid,56%,mp 210- 212 ℃; 1H NMR (600 MHz,DMSO-d6):δ9.28 (brs,2H,pyrrole-NH), 8.89 (brs,2H,pyrrole-NH),7.18 (t,1H,J = 7.8 Hz,ArH),6.86-6.82 (m,2H,ArH),6.63 (d,1H,J = 7.2 Hz,ArH),5.76 (d,4H,J = 12 Hz,bpyrrole- H),5.67 (s,4H,β-pyrrole-H),4.20 (brs,2H,CH2),2.03-2.00 (m,2H,CH2),2.00-1.90 (m,4H,CH2),1.89 (s,3H,CH3),1.87-1.70 (m,4H,CH2),1.70-1.65 (m,2H,CH2),0.63 (d,12H,J = 6.0 Hz,CH3), 0.58 (t,3H,J = 7.2 Hz,CH3),0.44 (t,3H,J = 7.2 Hz,CH3); 13C NMR (150 MHz,DMSO-d6):δ169.1,156.5,137.7,136.2,135.7,135.8, 128.8,127.7,121.1,114.5,104.3,103.4,103.1,103.0,65.8,51.5, 42.8,42.6,41.8,29.7,28.0,25.6,8.6,8.0,7.8; IR (KBr,cm-1) ν:3906, 3438,3362,3108,2952,2871,2665,1766,1573,1433,1208,1128, 1049,767,707,527; ESI-HRMS Calcd. for C42H54N4NaO3 ([M+Na]+) 685.4094,found 685.4088.
2.3. Synthesis of 5-methyl-10,10,15,15,20,20-hexaethyl-5-(2-(2- aminoethylamino)-2-oxoethoxyphenyl)calix[4]pyrrole (3)Calix[4] pyrroleoxyacetate 3 (1.0 mmol,0.785 g) was dissolved in 30 mL of ethanol,then excess ethylenediamine (10.0mL)was added. The mixture was refluxed overnight. The mixture was poured with vigorous stirring into a large amount of water (100mL) with saturated salt. The resulting precipitate was collected by filtration and washed with little cold ethanol to give the pure product,white solid,72%,mp130-132 ℃; 1HNMR(400MHz,CDCl3):δ7.29 (brs,2H, pyrrole-NH),7.19 (d,1H,J = 12.0 Hz,ArH),7.07 (brs,2H,pyrrole-NH), 6.85 (t,1H,J = 10.8 Hz,ArH),6.75 (d,2H,J = 13.8 Hz,ArH),6.35 (t,1H, J = 9.6 Hz,NH),5.94 (t,2H,J = 2.4 Hz,β-pyrrole-H),5.89 (t,4H, J = 19.6 Hz,β-pyrrole-H),5.70 (brs,2H,β-pyrrole-H),4.36 (s,2H, CH2),3.16 (dd,2H,J = 12.0,6.0 Hz,CH2),2.68 (t,2H,J = 9.0Hz,CH2), 1.98 (s,3H,CH3),1.89-1.68 (m,12H,CH2),0.65-0.58 (m,18H,CH3); 13CNMR(100 MHz,CDCl3):δ168.8,154.6,136.0,135.2,129.3,128.4, 121.4,111.3,105.2,104.7,104.3,66.5,43.5,42.9,42.0,41.5,28.9,28.5, 25.7,8.0; IR (KBr,cm-1) ν:3852,3755,3425,3344,3110,2962,2878, 2315,1675,1540,1443,1375,1223,1125,1046,927,769,705,575; ESI-HRMS Calcd. for C43H59N6O2 ([M+H+]) 691.4700,found 691.4694.
2.4. Synthesis of calix[4]pyrrole mono-Schiff bases 4a-4eA suspension of calix[4]pyrrole 3 (0.5 mmol) and salicylladehyde or its derivatives (1.2 mmol) in 20 mL of ethanol was added two drops of concentrated hydrochloric acid. The mixture of was refluxed for 12 h. The resulting precipitate was collected by filtration and washed with cold ethanol to give pure product 4a-4e for analysis.
5-Methyl-10,10,15,15,20,20-hexaethyl-5-(2-(2-(2-hydroxybenzylideneamino) ethylamino)-2-oxoethoxyphenyl)calix[4]- pyrrole (4a): Slight yellow solid,74%,mp 190-192 ℃; 1H NMR (400 MHz,CDCl3):δ13.05 (brs,1H,OH),8.11 (s,1H,CH55N),7.28 (t, 1H,J = 12.0 Hz,ArH),7.19 (s,2H,pyrrole-NH),7.13 (t,1H, J = 12.0 Hz,ArH),7.05 (d,1H,J = 11.4 Hz,ArH),6.96 (s,1H,ArH), 6.93 (s,2H,pyrrole-NH),6.83-6.75 (m,2H,ArH),6.69 (d,1H, J = 12.0 Hz,ArH),6.56 (d,1H,J = 11.4 Hz,ArH),6.35 (s,1H,NH), 5.93 (s,2H,β-pyrrole-H),5.88 (s,4H,β-pyrrole-H),5.57 (brs,2H,bpyrrole- H),4.34 (s,2H,CH2),3.64 (brs,2H,CH2),3.44 (brs,2H,CH2), 1.85 (s,3H,CH3),1.84-1.53 (m,12H,CH2),0.61 (s,18H,CH3); 13C NMR (100 MHz,CDCl3):δ168.8,166.4,158.1,154.4,135.8,135.3, 132.6,131.6,129.3,128.2,121.4,118.5,116.9,110.9,105.2,104.7, 104.2,66.2,58.6,43.5,42.8,39.4,28.9,28.4,25.0,8.1,8.0,7.9; IR (KBr,cm-1) ν:3898,3852,3796,3731,3417,3340,3101, 2969,1671,1579,1537,1448,1281,1208,1128,1045,765,586; ESI-HRMS Calcd. for C50H62N6NaO3 ([M+Na]+) 817.4781,found 817.4776.
5-Methyl-10,10,15,15,20,20-hexaethyl-5-(2-(2-(5-chloro-2- hydroxybenzylideneamino)ethylamino)-2-oxoethoxy)phenyl)- calix[4]pyrrole (4b): Yellow solid,83%,mp 212-214 ℃; 1H NMR (400 MHz,CDCl3):δ13.15 (brs,1H,OH),7.96 (s,1H,CH55N),7.22 (d,1H,J = 9.0 Hz,ArH),7.17 (s,2H,pyrrole-NH),7.14 (s,1H,ArH), 6.96 (s,1H,ArH),6.92 (s,1H,ArH),6.90 (s,2H,pyrrole-NH),6.84 (t, 1H,J = 7.2 Hz,ArH),6.68 (d,1H,J = 8.2 Hz,ArH),6.56 (d,1H, J = 11.4 Hz,ArH),6.30 (t,1H,J = 7.8 Hz,NH),5.92 (s,2H,β-pyrrole- H),5.88 (s,4H,β-pyrrole-H),5.50 (brs,2H,β-pyrrole-H),4.35 (s,2H,CH2),3.65 (brs,2H,CH2),3.44 (brs,2H,CH2),1.86 (s,3H,CH3), 1.83-1.78 (m,12H,CH2),0.64-0.58 (m,18H,CH3); 13C NMR (100 MHz,CDCl3):δ168.8,165.2,159.5,154.4,135.6,135.3,132.3, 130.7,129.3,128.1,123.0,121.5,118.9,118.4,110.4,105.2,104.8, 104.1,66.0,58.9,43.4,42.8,39.2,28.9,28.4,28.2,24.8,8.0; IR (KBr, cm-1) ν:3823,3758,3696,3426,3343,3110,2960,2877,2314, 1671,1538,1478,1371,1279,1207,1120,1045,769,702,578; ESI-HRMS Calcd. for C50H61ClN6NaO3 ([M+Na]+) 851.4391,found 851.4386.
5-Methyl-10,10,15,15,20,20-hexaethyl-5-(2-(2-(5-bromo-2- hydroxybenzylideneamino)ethylamino)-2-oxoethoxy)phenyl)- calix[4]pyrrole (4c): Yellow solid,45%,mp 216-218 ℃; 1H NMR (400 MHz,CDCl3):δ13.10 (brs,1H,OH),7.93 (s,1H,CH55N),7.35 (d,1H,J = 12.6 Hz,ArH),7.19 (s,2H,pyrrole-NH),7.15 (d,1H, J = 11.4 Hz,ArH),7.08 (s,1H,ArH),6.91 (s,2H,pyrrole-NH),6.88- 6.81 (m,2H,ArH),6.68 (d,1H,J = 12.0 Hz,ArH),6.56 (d,1H, J = 10.8 Hz,ArH),6.29 (s,1H,NH),5.92 (s,2H,β-pyrrole-H),5.87 (s, 4H,β-pyrrole-H),5.49 (brs,2H,β-pyrrole-H),4.35 (s,2H,CH2),3.63 (s,2H,CH2),3.43 (brs,2H,CH2),1.86 (s,3H,CH3),1.85-1.62 (m, 12H,CH2),0.65-0.61 (m,18H,CH3); 13C NMR (100 MHz,CDCl3): d 168.8,165.2,160.0,154.3,135.6,135.2,133.7,129.3,128.1,121.5, 119.5,118.9,110.4,109.8,105.2,104.7,104.1,66.0,58.9,43.4,42.8, 39.2,28.9,28.4,28.2,24.8,8.0; IR (KBr,cm-1) ν:3849,3425,3354, 2963,2878,2313,1668,1577,1536,1477,1371,1280,1206,1124, 1044,767,700,579; ESI-HRMS Calcd. for C50H61BrN6NaO3 ([M+Na]+) 851.4391,found 851.4386.
5-Methyl-10,10,15,15,20,20-hexaethyl-5-(2-(2-(3,5-di-tertbutyl- 5-bromo-2-hydroxybenzylideneamino)ethylamino)-2- oxoethoxy) phenyl)calix[4]pyrrole (4d): Light yellow solid,79%, mp 128-130 ℃; 1H NMR (400 MHz,CDCl3):δ13.51 (brs,1H,OH), 8.20 (s,1H,CH55N),7.36 (s,1H,NH),7.17 (s,2H,pyrrole-NH),7.07 (s,1H,ArH),6.97 (s,1H,ArH),6.92 (s,2H,pyrrole-NH),6.75 (t,1H, J = 8.0 Hz,ArH),6.68 (d,1H,J = 7.2 Hz,ArH),6.60 (d,1H,J = 8.0 Hz, ArH),6.36 (s,1H,ArH),5.93 (s,2H,β-pyrrole-H),5.87 (s,4H,bpyrrole- H),5.59 (brs,2H,β-pyrrole-H),4.34 (s,2H,CH2),3.62 (brs,2H,CH2),3.42 (brs,2H,CH2),1.83 (s,3H,CH3),1.76-1.56 (m,12H, CH2),1.39 (s,9H,CH3),1.27 (s,9H,CH3),0.60 (s,18H,CH3). 13CNMR (100 MHz,CDCl3):δ168.7,167.4,157.9,135.7,135.3,129.3,128.2, 127.3,125.7,121.3,117.5,110.9,105.3,104.8,104.3,66.2,58.5, 42.8,34.9,31.4,29.4,28.9,28.6,28.4,25.4,8.1,8.0,7.9; IR (KBr, cm-1) ν:3850,3732,3432,3347,2919,1682,1632,1580,1534, 1449,1371,1208,1123,1045,768,705,532; ESI-HRMS Calcd. for C58H78N6NaO3 ([M+Na]+) 929.6033,found 929.6028.
5-Methyl-10,10,15,15,20,20-hexaethyl-5-(2-(2-hydroxynaphthalen- 1-yl)methyleneamino)-2-oxoethoxy)phenyl)calix[4]- pyrrole (4e): Yellow solid,77%,mp118-120 ℃; 1HNMR (400 MHz, CDCl3):δ12.98 (brs,1H,OH),8.72 (s,1H,CH55N),7.81 (d,1H, J = 8.4 Hz,ArH),7.69 (d,1H,J = 9.2 Hz,ArH),7.63 (d,1H,J = 8.0 Hz, ArH),7.44 (t,1H,J = 7.6 Hz,NH),7.27 (d,1H,J = 7.4 Hz,ArH),7.21 (s,2H,pyrrole-NH),7.00 (d,1H,J = 9.2 Hz,ArH),6.93 (s,2H, pyrrole-NH),6.91 (s,1H,ArH),6.63 (d,1H,J = 8.0 Hz,ArH),6.56 (t, 1H,J = 7.2 Hz,ArH),6.49 (d,1H,J = 80,Hz,ArH),6.43 (t,1H, J = 6.0 Hz,ArH),5.94 (t,2H,J = 2.8 Hz,β-pyrrole-H),5.88 (t,2H, J = 2.8 Hz,β-pyrrole-H),5.86 (t,2H,J = 2.8 Hz,β-pyrrole-H),5.61 (brs,2H,β-pyrrole-H),4.35 (s,2H,CH2),3.73 (s,2H,CH2),3.45 (d, 2H,J = 5.6 Hz,CH2),1.87-1.84 (m,2H,CH2),1.83 (s,3H,CH3),1.81- 1.64 (m,10H,CH2),0.69-0.51 (m,18H,CH3); 13C NMR (150 MHz CDCl3):δ169.1,159.8,154.3,136.5,136.1,136.1,135.9,133.3, 129.4,129.1,128.3,127.8,126.7,123.2,122.9,121.3,118.4,110.9, 105.1,104.8,104.1,66.2,58.3,54.2,43.5,42.9,39.7,29.0,28.8, 28.4,25.1,18.3,8.1,8.0,7.9; IR (KBr,cm-1) ν:3815,3754,3694, 3333,2966,1688,1630,1536,1438,1353,1240,1126,1046,845, 767,592,517; ESI-HRMS Calcd. for C54H64N6NaO3 ([M+Na]+) 867.4938,found 867.4932.
2.5. Synthesis of metal complexes of calix[4]pyrrole Schiff basesA mixture of calix[4]pyrrole Schiff base 4b (0.1 mmol) and transition metal acetate (0.5 mmol) in 30 mL of ethanol was refluxed over night. The resulting precipitate was filtrated and washed with ethanol and water to give the complex. Ni complex (4b-Ni): Dark green solid,mp 156-158 ℃; IR (KBr,cm-1) ν:3433, 3348,3108,2966,2932,2876,1679,1616,1578,1532,1464,1426, 1390,1324,1285,1232,1205,1133,1108; ESI-HRMS Calcd. for C50H60N6O3ClNi ([NiL+H]+,L=4b) 885.3769; found 885.3763. Copper complex (4b-Cu): yellow solid,mp 147-149 ℃; IR (KBr, cm-1) ν:3435,3348,3106,2966,2934,2876,1679,1624,1574, 1529,1464,1420,1389,1325,1284,1235,1201,1133,1047,927, 826,768,707,663,552; ESI-HRMS Calcd. for C50H60ClCuN6O3 ([CuL+H]+,L = 4b) 890.3633,found 890.3600.
3. Results and discussionThe synthetic route for the calix[4]pyrrole mono-Schiff bases is illustrated in Scheme 1. According to the known synthetic method for meso-substituted calix[4]pyrroles by macrocyclization reaction of ketones with pyrrole [31, 32]. The condensation reaction of 5,50-diethyldipyrromethane with o-hydroxyacetophenone in methanol in the presence of methanesulfonic acid afforded a mixture of mono-(2-hydroxyphenyl)calix[4]pyrrole,di-(2- hydroxyphenyl)calix[4]pyrrole and tri-(2-hydroxyphenyl)calix[ 4]pyrrole. The desired 5-methyl-10,10,15,15,20,20-hexaethyl- 5-(2-hydroxyphenyl)calix[4]pyrrole (1) can be separated in about 15% yield after column chromatography. Then,calix[4]pyrrole (1) was alkylated with methyl a-chloroacetate in refluxing acetone with potassium carbonate as base and potassium iodide as catalyst. The corresponding calix[4]pyrroleoxyacetate 2 can be prepared in 56% yield. The ammonolysis of the ester group in calix[4]pyrroleoxyacetate 2 with excess ethylenediamine in refluxing ethanol gave the desired amides derivatives bearing with free v-amino group 3. Finally,the condensation of calix[4]pyrrole amide 3 with salicylaldehyde and its 5-chloro, 5-bromo,3,5-dibutyl derivatives and 2-hydroxynaphthaldehyde in ethanol gave the expected calix[4]pyrrole Schiff bases 4a-e in satisfactory yields. The structures of the prepared calix[4]pyrrole Schiff bases 4a-e were characterized by IR,HRMS,1H NMR and 13C NMRspectra. For example,1H NMRspectra of calix[4]pyrrole Schiff base 4b displays a broad peak at 13.05 ppm for OH group,a singlet at 8.11 ppm for CH=N unit,a singlet at 4.34 ppm for CH2 unit and two broad singlets at 3.75,3.67 ppm for two ethylene units.
|
Download:
|
| Scheme 1.Synthetic route for calix[4]pyrrole mono-Schiff bases 4a-e. | |
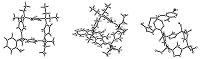
The single crystal structures of three calix[4]pyrroles 1,2 and 4b were determined by X-ray diffraction method (Fig. 1). The structures were solved by direct methods using crystal structure and refined by the full-matrix least squares method using SHELXL97 [34]. calix[4]pyrroles 1,2 and 4b adopts same 1,3- alternate configuration,in which the two pyrrole rings at 1,3- positions exist to one direction,while the other two pyrrole rings at 2,4-positions stand to another direction. In the molecular structure of calix[4]pyrrole 2,two N-H···O H-bonds are formed between the two pyrroles at 1,3-positions and the two oxygen atoms inoxyacetate,which causes the chain of methyl oxyacetate exists on the top of cavity of calix[4]pyrrole core. In the calix[4]pyrrole 4b, the open-chain of oxyacetamide unit stretches to the outside of calix[4]pyrrole core,while the 2-hydroxy-5-chlorobenzylidene group is partially above the cavity of calix[4]pyrrole core.
|
Download:
|
| Fig. 1.Molecular structure of calix[4]pyrrole 1,2,4b. | |
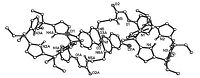
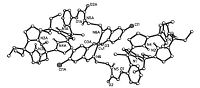
The coordination reactions of calix[4]pyrrole Schiff bases to transition metal ions were investigated. After refluxing the ethanol solution of calix[4]pyrrole mono-Schiff bases 4a-4e with common transition metal acetates for several hours,the solid products of transition metal complexes were easily obtained in high yields. We were pleased to obtain the suitable single crystals of 4b-Ni and 4b- Cu for X-ray diffraction determination (Figs. 2 and 3). In the complexes 4b-Ni and 4b-Cu,it is interesting to find that the two calix[4]pyrrole cores display a unusual partial cone conformation, in which the three pyrrole units exist upside and the fourth pyrrole unit exists upside down,which is different to the 1,3-alternate configuration of the precursors 1,2 and 4b. In the complexes 4b-Ni and 4b-Cu,the calix[4]pyrrole skeleton only acts as backbone, while the functionalized Schiff base acts as chelated ligand to coordinate to Ni2+ or Cu2+. As each Schiff base moiety coordinated to one metal ion,the copper complexes are formed with 2:1 ratio of metal ion to ligands,in which the metal ion is coordinated by two phenolic oxygen atoms and two imino nitrogen atoms to form an approximately square-planar geometry.
|
Download:
|
| Fig. 2.Molecular structure of calix[4]pyrrole 4b-Ni. | |
|
Download:
|
| Fig. 3.Molecular structure of calix[4]pyrrole 4b-Cu. | |
In summary,we have successfully prepared a series of calix[4]pyrrole meso-substituted Schiff bases by using 5-methyl- 10,10,15,15,20,20-hexaethyl-5-(2-hydroxyphenyl)calix[4]pyrrole as starting material. Furthermore,the nickel and copper complexes of calix[4]pyrrole meso-substituted Schiff base with 1:2 stoichiometry were also successfully obtained. The crystal structures of the calix[4]pyrroles derivatives and their metal complexes were determined by X-ray diffraction. It is interesting to find that calix[4]pyrrole cores adopts a unusual partial cone conformation in metal complexes,while they usually display 1,3-alternate configuration in the precursor ligands.
Supporting informationThe detailed spectroscopic data including crystallographic data (CIF) of all new compounds are available. Single crystal data for compounds 1 (CCDC 1018497),2 (CCDC 1018498),4b (CCDC1018499),4β-Cu (CCDC 1018500),4β-Ni (CCDC1018501) have been deposited in the Cambridge Crystallographic Data Center.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (No. 21172190) and the Priority Academic Program Development of Jiangsu Higher Education Institutions. We also thank Analysis and Test Center of Yangzhou University providing instruments for analysis.
| [1] | P.A. Gale, P. Anzenbacher, J.L. Sessler, Calixpyrroles II, Coord. Chem. Rev. 222 (2001) 57-102. |
| [2] | P.A. Gale, C.C. Tong, C.J.E. Haynes, et al., Octafluorocalix[4]pyrrole: a chloride/bicabonate antiport agent, Coord. Chem. Rev. 132 (2010) 3240-3241. |
| [3] | P.A. Gale, S.E. García-Garrido, J. Garric, Anion receptors based on organic frameworks: highlights from 2005 and 2006, Chem. Soc. Rev. 37 (2008) 151-190. |
| [4] | A.E. Hargrove, S. Nieto, T.Z. Zhang, J.L. Sessler, E.V. Anslyn, Artificial receptors for the recognition of phosphorylated molecules, Chem. Rev. 111 (2011) 6603-6782. |
| [5] | T.G. Levitskaia, M. Marquez, J.L. Sessler, et al., Fluorinated calixpyrroles: anionbinding extractants that reduce the Hofmeister bias, Chem. Commun. 17 (2003) 2248-2249. |
| [6] | B. Taner, Novel vic-dioxime ligand containing calix[4]pyrrole moiety: synthesis, characterization, anion binding studies and complexation with Ni(II), J. Incl. Phenom. Macrocycl. Chem. 79 (2014) 75-81. |
| [7] | H. Miyaji, H.K. Kim, E.K. Sim, et al., Coumarin-strapped calix[4]pyrrole: a fluorogenic anion receptor modulated by cation and anion binding, J. Am. Chem. Soc. 127 (2005) 12510-12512. |
| [8] | B. Taner, P. Deveci, S. Bereket, A.O. Solak, E. Özcan, The first example of calix[4]-pyrrole functionalized vic-dioxime ligand: synthesis, characterization, spectroscopic studies and redox properties of the mononuclear transition metal complexes, Inorg. Chim. Acta 363 (2010) 4017-4023. |
| [9] | G. Cafeo, F.H. Kohnke, M.F. Parisi, et al., The elusive b-unsubstituted calix[5]pyrrole finally captured, Org. Lett. 4 (2002) 2695-2697. |
| [10] | J.L. Sessler, D.Q. An, W.S. Cho, et al., Anion-binding behavior of hybrid calixpyrroles, J. Org. Chem. 70 (2005) 1511-1517. |
| [11] | B. Mokhtari, K. Pourabdollah, Analytical applications of nano-baskets of calix[4]-pyrroles, J. Incl. Phenom. Macrocycl. Chem. 77 (2013) 23-31. |
| [12] | K.D. Bhatt, D.J. Vyas, B.A. Makwana, S.M. Darjee, V.K. Jain, Highly stable water dispersible calix[4]pyrrole octa-hydrazide protected gold nanoparticles as colorimetric and fluorometric chemosensors for selective signaling of Co(II) ions, Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 121 (2014) 94-100. |
| [13] | A. Aydogan, A. Akar, Tri-and pentacalix[4]pyrroles: synthesis, characterization and their use in the extraction of halide salts, Chem. Eur. J. 18 (2012) 1999-2005. |
| [14] | G. Cafeo, G. Gattuso, F.H. Kohnke, et al., Host-guest chemistry of aromatic-amidelinked bis-and tris-calix[4]pyrroles with bis-carboxylates and citrate anion, Chem. Eur. J. 20 (2014) 1658-1668. |
| [15] | J.L. Sessler, E. Tomat, Transition-metal complexes of expanded porphyrins, Acc. Chem. Res. 40 (2007) 371-379. |
| [16] | Y. Matano, H. Imahori, Phosphole-containing calixpyrroles, calixphyrins, and porphyrins: synthesis and coordination chemistry, Acc. Chem. Res. 42 (2009) 1193-1204. |
| [17] | J. Jubb, C. Floriani, A. Chiesi-Villa, C. Rizzoli, Redox chemistry of meso-octaethylporphyrinogen: formation and opening of a cyclopropane ring, J. Am. Chem. Soc. 114 (1992) 6571-6573. |
| [18] | S. De Angelis, E. Solari, C. Floriani, A. Chiesi-Villa, C. Rizzoli, Oxidation of metalmeso-octaethylporphyrinogen complexes leading to novel oxidized forms of porphyrinogen other than porphyrins. 1. The redox chemistry of nickel(I1)-and copper (11)-meso-octaethylporphyrinogen complexes occurring with the formation and cleavage of a cyclopropane unit, J. Am. Chem. Soc. 116 (1994) 5691-5701. |
| [19] | T. Nakabuchi, Y. Matano, H. Imahori, Synthesis, structures, and coordinating properties of phosphole-containing hybrid calixpyrroles, Organometallics 27 (2008) 3142-3152. |
| [20] | V. Blangy, C. Heiss, V. Khlebnikov, et al., Synthesis, structure, and complexation properties of partially and completely reduced meso-octamethylporphyrinogens (calix[4]pyrroles), Angew. Chem. Int. Ed. 48 (2009) 1688-1691. |
| [21] | G.B. Deacon, M.G. Gardiner, P.C. Junk, J.P. Townley, J. Wang, Rare-earth metalation of calix[4]pyrrole/calix[4]arene free of alkali-metal companions, Organometallics 31 (2012) 3857-3864. |
| [22] | E. Askarizadeh, A.M.J. Devoille, D.M. Boghaei, A.M.Z. Slawin, J.B. Love, Ligand modifications for tailoring the binuclear microenvironments in Schiff-base calixpyrrole pacman complexes, Inorg. Chem. 48 (2009) 7491-7500. |
| [23] | J.B. Love, A macrocyclic approach to transition metal and uranyl pacman complexes, Chem. Commun. (2009) 3154-3165. |
| [24] | E. Askarizadeh, S.B. Yaghoo, D.M. Boghaei, A.M.Z. Slawinc, J.B. Love, Fluidization characteristics of printed circuit board plastic particles with different sizes, Chem. Commun. 46 (2010) 710-712. |
| [25] | Q.J. Pan, G. Schreckenbach, P.L. Arnold, J.B. Love, Theoretical predictions of cofacial bis(actinyl) complexes of a stretched Schiff-base calixpyrrole ligand, Chem. Commun. 47 (2011) 5720-5722. |
| [26] | B. Taner, P. Deveci, E. Özcan, A.O. Solak, A novel vic-dioxime ligand and its Ni(II), Cu(II) and Co(II) complexes containing calix[4]pyrrole moiety: synthesis, characterization and redox properties, J. Incl. Phenom. Macrocycl. Chem. 74 (2012) 391-396. |
| [27] | G. Cafeo, G. Carbotti, A. Cuzzola, et al., Drug delivery with a calixpyrrole-trans-Pt(II) complex, J. Am. Chem. Soc. 135 (2013) 2544-2551. |
| [28] | C.G. Yan, L. Li, W.L. Liu, Metallic macrocycle with a 1,3-alternate calix[4]arene salicylideneamine ligand, J. Coord. Chem. 62 (2009) 2118-2124. |
| [29] | L. Liu, K. Huang, C.G. Yan, Syntheses, reactions and crystal structures of 1,3-alternate p-tert-butylthiacalix[4]arene esters and amides, J. Incl. Phenom. Macrocycl. Chem. 66 (2010) 349-355. |
| [30] | J. Sun, L.L. Zhang, Y. Yao, C.G. Yan, Synthesis, crystal structures and complexing properties of tetramethoxyresorcinarene functionalized tetraacylhydrazones, J. Incl. Phenom. Macrocycl. Chem. 79 (2014) 485-494. |
| [31] | Y. Han, G.L. Wang, J.J. Sun, J. Sun, C.G. Yan, Synthesis and crystal structure of 15α, 20α-di(4-hydroxylphenyl)calix[4]pyrroles and 10α,20β-di(4-hydroxylphenyl)-calix[4]pyrroles, Tetrahedron 69 (2013) 10604-10609. |
| [32] | Y. Han, J.J. Sun, G.L. Wang, C.G. Yan, Synthesis and properties of functionalized schiff bases of 5α, 10α-di(4-hydroxylphenyl)calix[4]pyrrole and 5α,15β-di(4-hydroxylphenyl)calix[4]pyrrole, Chem. Res. Chin. Univ. 30 (2014) 919-924. |
| [33] | Y. Han, J.J. Sun, G.L. Wang, C.G. Yan, Synthesis, crystal structure and complexing properties of calix[4]pyrrole 10a,20a-disubstituted Schiff bases and urea derivatives, J. Mol. Struct. 1083 (2015) 300-310. |
| [34] | G.M. Sheldrick, SHELX9Z Structure Determination Programs, University of Göttingen, Göttingen, 1997. |




