Redox-active organic and organometallic compounds are important materials for molecular electronics. They are useful in a wide range of applications,such as molecular wires [1],mixedvalent chemistry [2, 3],ion sensing [4, 5],electrochromism [6, 7], information storage [8],and the fundamental study of electrochemistry [9],among others [10, 11]. Most reported redox-active materials contain one or two redox sites. Materials with more than three redox sites and redox states are appealing for their applications in molecular electronics.
We have previously reported that the combination of cyclometalated ruthenium complexes [12] and redox-active triarylamines can generate new hybrid systems with multi-stage nearinfrared (NIR) electrochromism with low-potential electrochemical inputs [13]. This is a result of the strong electronic coupling between two redox species. When a diruthenium component was used as the conjugated bridge to connect two triarylamines,a [N-Ru-Ru-N] system with multiple redox processes was obtained [14]. We present herein the synthesis and electrochemical and spectroscopic study of a related [N-Os-Os-N] system using a cyclometalated diosmium component as the bridge unit. The replacement of ruthenium by osmium is expected to further decrease the involving redox potentials [15]. In addition,osmium (Ⅲ) complexes are known to possess characteristic d-d marker absorption bands [16],which are believed to add new absorption features to these multi-center materials.
2. ExperimentalNMR spectra were recorded in the designated solvent on Bruker Avance 400 MHz spectrometer. Spectra are reported in ppm values from residual protons of deuterated solvent. Mass data were obtained with a Bruker Daltonics Inc. Apex Ⅱ FT-ICR or Autoflex Ⅲ MALDI-TOF mass spectrometer. The matrix for MALDI-TOF measurement is a-cyano-4-hydroxycinnamic acid. Microanalysis was carried out using Flash EA 1112 or Carlo Erba 1106 analyzer at the Institute of Chemistry,Chinese Academy of Sciences.
Synthesis of 1(PF6)2: To a 100 mL flask were tetra(pyrid-2- yl)pyrazine (tppz,0.10 mmol,38.8 mg),(NH4)2OsCl6 (0.24 mmol, 88.4 mg),and 50 mL dry DMF. The mixture was stirred at 120 ℃ for 3 h. After cooling to room temperature,the solvent was removed under reduced pressure. To the residue were added proper amount of ether. The resulting precipitate was collected after filtration and washing with ethyl ether to give 96 mg of [OsCl3(tppz)OsCl3] as a black solid in 99% yield. This material was used without further purification for next transformation. To 5 mL of ethylene glycol were added the above-prepared [OsCl3(tppz)OsCl3] (0.020 mmol, 19.6 mg) and 1-(di-p-anisylamino)-3,5-di(pyrid-2-yl)benzene (dadpb,0.042 mmol,19.3 mg) [17]. The mixture was heated under microwave condition (power = 375 W) for 30 min. After cooling to room temperature,the system was treaded with 5 mL of aqueous solution containing 200 mg of KPF6. After filtration and washing successively with water and ether,the obtained solid was subjected to column chromatography on silica gel (eluent: CH3CN/H2O/saturated aqueous KNO3,30/1/0.05),followed by anion exchange using KPF6,to give 13.2 mg of 1(PF6)2 as a black solid in 30% yield. 1H NMR (300 MHz,acetone-d6): d 9.31 (d,4H, J = 6.3 Hz),8.49 (d,4H,J = 6.3 Hz),8.25-8.28 (m,8H,J = 7.5 Hz), 8.07 (t,4H,J = 11.7 Hz) 7.98 (t,4H,J = 11.7 Hz),7.81 (d,4H, J = 4.2 Hz),7.60-7.65 (m,12H,J = 13.8 Hz),7.26 (t,4H,J = 10.2 Hz), 7.19 (d,8H,J = 6.6 Hz),3.924 (s,12H). 13C NMR (100 MHz, acetone-d6): d 170.81,160.67,157.33,155.93,153.71,143.44, 141.80,136.88,134.66,128.77,128.33,125,17,122.86,122.42, 121.13,115.33. MALDI-TOF-MS (m/z): 1831.2 [M-PF6]+, 1686.3 [M-2PF6]+. Anal. Calcd. for C84H64F12N12O4Os2P2·3H2O: C,49.70; H,3.48; N,8.28. Found: C,49.72; H,3.25; N,8.53.
Electrochemical measurements: All CV and DPV measurements were taken using a CHI 660D potentiostat with one-compartment electrochemical cell under an atmosphere of nitrogen. All measurements were carried out in 0.1 mol/L nBu4NClO4/CH3CN. CV was measured at a scan rate of 100 mV/s. The working electrode was a glassy carbon with a diameter of 3.0 mm. The electrode was polished prior to use with 0.05 μm alumina and rinsed thoroughly with water and acetone. A large area platinum wire coil was used as the counter electrode. All potentials are referenced to a Ag/AgCl electrode in saturated aqueous NaCl without regard for the liquid junction potential. The potential vs. Fc0/+ can be estimated from that vs. Ag/AgCl by subtracting 0.45 V.
Spectroscopic measurements: During the measurement with chemical oxidation,different equivalent of oxidant (cerium ammonium nitrate,CAN,in CH3CN) were added to a solution of the compound in study with constant concentration. The obtained solution was measured by the PE Lambda 750 UV/vis/NIR spectrophotometer.
DFT calculations: DFT calculations are carried out using the B3LYP exchange correlation functional [18] and implemented in the Gaussian 09 package. The electronic structures of complexes were determined using a general basis set with the Los Alamos effective core potential LANL2DZ basis set for ruthenium [19], and 6-31G* for other atoms. Solvation effects in CH3CN are included with the conductor-like polarizable continuum model (CPCM).
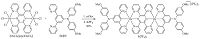
3. Results and discussionThe desired compound 1(PF6)2 was synthesized as outlined in Scheme 1. The reaction of [OsCl3(tppz)OsCl3],which was prepared from tetra(pyrid-2-yl)pyrazine (tppz) and (NH4)2OsCl6,with 1-(dip- anisylamino)-3,5-di(pyrid-2-yl)benzene (dadpb) [17],followed by anion exchange using KPF6,gave 1(PF6)2 in 30% yield. The TOFMALDI mass spectrum shows signals of 1(PF6)2 after loss of one or two anions. This compound was fully characterized by NMR and microanalysis data.
|
Download:
|
| Scheme 1.Synthesis of 1(PF6)2. | |
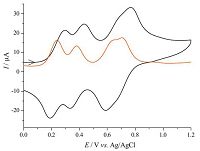
The anodic cyclic voltammogram (CV) and differential pulse voltammogram (DPV) of 1(PF6)2 shows four well-separated one-electron redox couples at +0.24,+0.38,+0.64,and +0.71 V vs. Ag/AgCl (Fig. 1). In comparison,the previously reported ruthenium analogous,[N-Ru-Ru-N],displays similar four waves at +0.38,+0.48,+0.83,and +0.91 V [14]. This indicates that the replacement of ruthenium by osmium can lead to a negative potential shift of these redox waves by 140-200 mV,which would be beneficial for the applications of these materials in electrochromic and memory devices. We previously found that materials with low redox potentials could result in devices with low operational voltage and long retention time [6, 8]. A related known cyclometalated di-Os complex bridged by the same bridging tppz, [(Mebip)Os(tppz)(Mebip)]2+ (Mebip = bis(N-methylbenzimidazolyl) pyridine),displays two Os(Ⅱ/Ⅲ) redox waves at -0.12 and +0.33 V vs. ferrocene+/0 (corresponding to +0.33 and +78 V vs. Ag/AgCl) [20]. It should also be mentioned that complex 1(PF6)2 is essentially nonemissive in dilute solution at room temperature.
|
Download:
|
| Fig. 1.CV and DPV of 1(PF6)2 at a glassy carbon electrode in 0.1 mol/L Bu4NClO4/CH3CN. | |
In accordance with the four consecutive redox processes, complex 1(PF6)2 shows four-step absorption spectral changes upon stepwise oxidations to transform 12+ into 16+ (Fig. 2). In the single oxidation of 1(PF6)2 with up to 1.0 equiv cerium ammonium nitrate (CAN) in CH3CN,the visible absorptions at 600 nm significantly decreased (Fig. 2a). At the same time,a strong absorption band at 1000 nm and a broad NIR band between 1500 and 2700 nm appeared. The new band at 1000 nm is ascribed to the charge resonance transition of the [N-Os] component. The NIR transition is due to the d-d transitions of osmium(Ⅲ) complexes [15, 16]. Similar absorptions have been observed for a previously reported cyclometalted monoosmium complex with a redox-active amine substituent [15]. In the double oxidation step with 2 equiv of CAN,the charge resonance transition at 1000 nmand the osmium(Ⅲ) d-d transitions continued to increase (Fig. 2b).

|
Download:
|
| Fig. 2.Absorption spectral changes of 1(PF6)2 upon stepwise oxidation with CAN in CH3CN. | |
During the triple oxidation step to transform 14+ into 15+ with 3 equiv of CAN (Fig. 2c),the charge resonance band decreased significantly and the osmium(Ⅲ) d-d transitions slightly shifted to a higher-energy region. In the meantime,a characteristic ammonium radical cation transition around 700 nm appeared [15]. In the forth-step oxidation with up to 4 equiv of CAN (Fig. 2d), this band continued to increase and the charge resonance band at 1000 nm gradually disappeared.
The above four-step spectral changes of 1(PF6)2 can basically be reproduced by spectroelectrochemical measurements by applying stepwise potentials from +0.10 V to +0.90 V,using a transparent indium-tin-oxide (ITO) glass as the working electrode (Fig. S1 in Supporting information). These spectral changes are reversible when a reverse potential was applied,indicating the potential application of this complex in electrochromic devices.
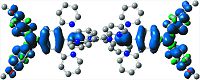
The appearance of four separated redox processes of 1(PF6)2 suggests that a possible electronic coupling between two distal amine sites through the diosmium bridge is present. However,no intervalence charge transfer (ⅣCT) transition can be established in the singly oxidized state 13+. The possible ⅣCT band could be buried in the osmium(Ⅲ) d-d transitions or localized in a lowerenergy region. Fig. 3 shows the DFT-calculated spin density population of 13+. The spin is distributed across both [N-Os] components,suggesting a delocalized character of the spin.
|
Download:
|
| Fig. 3.DFT-calculated Mulliken spin density population of 13+. Calculation method: UB3LYP/LANL2DZ/6-31-G*/CPCM. | |
A cyclometalted diosmiumcomplexwith two distal redox-active amine substituents have been synthesized and characterized. This complex shows four-step consecutive anodic processes due to the oxidations of two osmium-amine components. Accordingly, four-step absorption spectral changes have been observed upon stepwise oxidation,whichmakes it potentially useful in information storage and multi-stage electrochromic devices [6, 8].
AcknowledgmentsWe thank the National Natural Science Foundation of China (Nos. 21271176,91227104,21472196,and 21221002),the National Basic Research 973 program of China (No. 2011CB932301),the Ministry of Science and Technology of China (No. 2012YQ120060),and the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB 12010400) for funding support.
| [1] | H.M. Wen, Y. Yang, X.S. Zhou, et al., Electrical conductance study on 1,3-butadiyne-linked dinuclear ruthenium(Ⅱ) complexes within single molecule break junctions, Chem. Sci. 4 (2013) 2471-2477. |
| [2] | Y.P. Ou, J. Zhang, M. Xu, et al., Bridge-localized HOMO-binding character of divinylanthracene-bridged dinuclear ruthenium carbonyl complexes: spectroscopic, spectroelectrochemical, and computational studies, Chem. Asian J. 9 (2014) 1152-1160. |
| [3] | X. Xiao, M. Meng, H. Lei, C.Y. Liu, Electronic coupling and electron transfer between two dimolybdenum units spaced by a biphenylene group, J. Phys. Chem. C 118 (2014) 8308-8315. |
| [4] | Z.M. Su, H.M. Ye, X.X. Zhu, et al., A selective anion receptor based on 2, 2-diferrocenylpropane imidazolium sulphonate salt, J. Organomet. Chem. 750 (2014) 162-168. |
| [5] | P. Wang, T. Okamura, H.P. Zhou, W.Y. Sun, Y.P. Tian, Metal complex with terpyridine derivative ligand as highly selective colorimetric sensor for iron(Ⅲ), Chin. Chem. Lett. 24 (2013) 20-22. |
| [6] | B.B. Cui, C.J. Yao, J.N. Yao, Y.W. Zhong, Electropolymerized films as a molecular platform for volatile memory devices with two near-infrared outputs and long retention time, Chem. Sci. 5 (2014) 932-941. |
| [7] | A. Bolduc, C. Mallet, W.G. Skene, Survey of recent advances of in the field of π-conjugated heterocyclic azomethines as materials with tuneable properties, Sci. China Chem. 56 (2013) 3-23. |
| [8] | B.B. Cui, Z.P. Mao, Y.X. Chen, et al., Tuning of resistive memory switching in electropolymerized metallopolymeric films, Chem. Sci. 6 (2015) 1308-1315. |
| [9] | D.B. Xiang, H.B. Shao, Investigation of multilevel ion-pairing effect of triferrocenylmethane in organic phase, Chin. Chem. Lett. 25 (2014) 1379-1381. |
| [10] | K.Q. Wu, J. Guo, J.F. Yan, et al., Ruthenium(Ⅱ) bis(terpyridine) electron transfer complexes with alkynyl-ferrocenyl bridges: synthesis, structures, and electrochemical and spectroscopic studies, Dalton Trans. 41 (2012) 11000-11008. |
| [11] | F. Gao, L. Cui, W. Liu, et al., Seven-coordinate lanthanide sandwich-type complexes with a tetrathiafulvalene-fused Schiff base ligand, Inorg. Chem. 52 (2013) 11164-11172. |
| [12] | W.W. Yang, Y.W. Zhong, Cyclometalated ruthenium complexes of 1,2,3-triazolecontaining ligands: synthesis, structural studies, and electronic properties, Chin. J. Chem. 31 (2013) 329-338. |
| [13] | C.J. Yao, N.H. Nie, W.W. Yang, et al., Strongly coupled cyclometalated ruthenium-triarylamine hybrids: tuning electrochemical properties, intervalence charge transfer, and spindistribution by substituent effects, Chem. Eur. J.20 (2014)17466-17477. |
| [14] | C.J. Yao, Y.W. Zhong, J.N. Yao, Multi-center redox-active system: amine-amine electronic coupling through a cyclometalated bisruthenium segment, Inorg. Chem. 52 (2013) 4040-4045. |
| [15] | H.J. Nie, J.Y. Shao, C.J. Yao, Y.W. Zhong, Organic-inorganic mixed-valence systems with strongly-coupled triarylamine and cyclometalated osmium, Chem. Commun. 50 (2014) 10082-10085. |
| [16] | K.D. Demadis, G.A. Neyhart, E.M. Kober, P.S. White, T.J. Meyer, Intervalence transfer at the localized-to-delocalized, mixed-valence transition in osmium polypyridyl complexes, Inorg. Chem. 38 (1999) 5948-5959. |
| [17] | C.J. Yao, R.H. Zheng, Q. Shi, Y.W. Zhong, J.N. Yao, 1,4-Benzene-bridged covalent hybrid of triarylamine and cyclometalated ruthenium: a new type of organicinorganic mixed-valent system, Chem. Commun. 48 (2012) 5680-5682. |
| [18] | C. Lee, W.T. Yang, R.G. Parr, Development of the colle-salvetti correlation-energy formula into a functional of the electron density, Phys. Rev. B 37 (1988) 785-789. |
| [19] | P.J. Hay, W.R. Wadt, Ab initio effective core potentials for molecular calculations. potentials for K to Au including the outermost core orbitals, J. Chem. Phys. 82 (1985) 299-310. |
| [20] | T. Nagashima, T. Nakabayashi, T. Suzuki, et al., Tuning of metal-metal interactions in mixed-valence states of cyclometalated dinuclear ruthenium and osmium complexes bearing tetrapyridylpyrazine or -benzene, Organometallics 33 (2014) 4893-4904. |




