b Key Laboratory of Synthetic Chemistry of Natural Substances, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
The trifluoromethylated 2,3-dihydrofuran is a privileged structural motif present in some agrochemical candidates and bioactive compounds [1],thus their synthetic methodologies would be of potential application interest. The past decade has witnessed a significant advance on efficient construction of 2, 3-dihydrofuran structure [2],however,direct and convenient synthetic approaches for trifluoromethyl substituted dihydrofuran analogs from readily accessible starting materials and reagents under ambient conditions remain highly desirable [3].
Recently,nucleophilic phosphine or tertiary amine promoted cyclization reactions had proved themselves as a powerful tool to access synthetically valuable functionalized carbon or heterocycles [4]. When differently substituted allenoates reacted with CF3 aryl ketone,various trifluoromethylated oxygen heterocycles including dihydrofuran,dihydropyran and tetrahydrofuran can be synthesized respectively [3a, 5]. On the other hand,MBH carbonate has also been identified as versatile coupling partner in lieu of allenic ester to generate reactive zwitterion upon treatment of tertiary phosphine [6]. Very recently,we developed a chemoselective tandem reaction between g-nonsubstituted acrylate-derived MBH carbonates and trifluoromethyl aryl ketones (Scheme 1),whereas the tandem reaction sequence is terminated by a Wittig olefination. The reaction favored 5-exo-trig lactonization to give mono- or bicyclic vinyl γ-butenolide products [7]. Under certain circumstances, a small amount of CF3-bearing 2,3-dihydrofuran was also separated as byproduct concomitantly,which resulted from alternative 5-endo-trig ring-closing process. Conceivably,changing methyl ester group in MBH carbonates into acetyl group would enforce the reaction pathway toward 5-endo process,resulting in 2,3-dihydrofuran exclusively (Scheme 1). Herein we disclosed a phosphine-promoted [3 + 2] cyclization of nonsubstituted MVK (methyl vinyl ketone)-derived MBH carbonates and trifluoromethyl ketones to produce potentially useful CF3-containing 2, 3-dihydrofurans with completely controlled chemoselectivity in moderate yields.

|
Download:
|
| Scheme 1.Phosphine-promoted reactions of γ-nonsubstituted MBH carbonates and α-CF3 ketones. | |
MVK-derived MBH carbonate 2 was prepared according to literature [8]. Aryl CF3 ketones used in this work were purchased from commercial suppliers and were used without further purification. 1H NMR,19F and 13C NMR were recorded on Bruker 600 MHz or 400 MHz spectrometers in CDCl3 solution,using TMS as an internal standard. HRMS were performed using electrospray ionization (ESI) with Waters Synapt G2 Si. Melting points were determined on a SGW X-4 melting point apparatus and were uncorrected.
Initially,MVK-derived allylic carbonate 2 was exposed to 2 equiv. phenyl trifluoromethyl ketone 1a in the presence of various phosphine promoters with different electronic properties in CH2Cl2 at room temperature (Table 1). The anticipated 2, 3-dihydrofuran cycloadduct was isolated as the sole product in all cases and trialkylphosphine gave the best result,producing the corresponding product 3a in 57% isolated yield with excellent regioselectivity (Table 1,entries 1-4). Subsequent variation of reaction parameters,including ketone/carbonate molar ratio (Table 1,entries 5-7),phosphine loading (Table 1,entries 8-10) and temperature (Table 1,entry 12),did not avail further significant improvement for reaction efficiency. The cyclization seems to rely on the use of substoichiometric amount of Bu3P to maintain moderate conversion. In addition,the screening of solvents revealed that polar solvent DMF and protonic solvent ethanol resulted in either a small amount or trace of the desired cycloadduct,toluene was identified as the most suitable medium for the present cyclization. It should be mentioned that the standard conditions also produced some unidentified dark compound with high polarity inevitably.
|
|
Table 1 Optimization of reaction conditions.a |
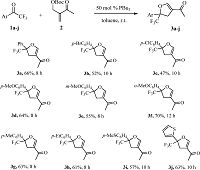
With the optimized reaction conditions in hand,we next attempted to briefly investigate the reaction scope (Scheme 2). CF3-activated ketones with either electron-donating,electronneutral or electron-withdrawing substituents at p- or m-position on aryl group all reacted uneventfully,affording a series of CF3- bearing 2,3-dihydrofuran in modest yields with excellent chemoand regioselectivities. In general,ketones with electron-donating and -neutral substituents usually delivered higher chemical yields than those with electron-withdrawing substituents. The sterically demanding 2-subtituted ketone 1f and heteroaryl ketone 1j were compatible with this cycloaddition protocol as well. However, aliphatic substrates were totally unsuitable (1,1,1-trifluoropropan- 2-one generally remained intact under our conditions). All new compounds were characterized by 1H,13C,19F NMR and HRMS spectra (Supporting information).
|
Download:
|
| Scheme 2.Substrate scope of [3 + 2] annulation reaction of MBH carbonate 2 and α-CF3 ketones 1. Reaction conditions: ketone 1 (0.1 mmol) and tert-butyl(2-methylene-3-oxobutyl)carbonate 2 (0.2 mmol) were stirred in the presence of n-Bu3P (0.05 mmol) in toluene at r.t. for 8–12 h. Yields refer to isolated yield. A small amount of uncharacterized compound was observed concurrently in the case of 3b, 3c or 3j. | |
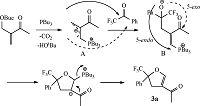
The mechanistic proposal for the exclusive formation of dihydrofuran is outlined in Scheme 3. The cyclization begins with the conversion of MVK-derived MBH carbonate 2 and PBu3 to allylic phosphorus ylide A via a well-defined SN2' addition/ deprotonation process. Then the in situ generated zwitterion A undergoes sterically favorable γ-addition to α-CF3 ketone to furnish intermediate B,otherwise the α-addition of allylic phosphorus ylide would lead to a Wittig olefination (γ-substituted allylic ylide would undergo a-addition,see Ref. [7b]). Subsequently, the betaine B cyclized exclusively in a 5-endo-trig fashion to produce 2,3-dihydrofuran 3a since the appendant acetyl group was not susceptible to alternative 5-exo lactonization.
|
Download:
|
| Scheme 3.Proposed reaction mechanism. | |
In conclusion,we disclosed a phosphine-promoted [3 + 2] cycloaddition reaction between MVK-derived MBH carbonate and aryl trifluoromethyl ketone,which provided a facile entry to CF3- substituted 2,3-dihydrofurans under ambient and metal-free conditions. The presented work demonstrated the reaction pattern of allylic phosphorus ylide with carbonyl compound can be successfully modulated to bypass their intrinsic tendency toward Wittig olefination.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21302034),and the Fundamental Research Funds for the Central Universities (No. 2013HGQC0028).
Appendix A. Supplementary dataSupplementary material related to this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.03.019.
| [1] | (a) J.C. Toueg, T. Smejkal, S.V. Wendeborn, et al., Preparation of dihydrofuran derivatives as insecticidal compounds, PCT Int. Appl. WO 2013026724 A1 20130228 (2013). (b) Y Shishido, H. Wakabayashi, H. Koike, et al., Discovery and stereoselective synthesis of the novel isochroman neurokinin-1 receptor antagonist ‘CJ-17,493', Bioorg. Med. Chem. 16 (2008) 7193-7205. |
| [2] | (a) T.G. Kilroy, T.P. O'Sullivan, P.J. Guiry, Synthesis of dihydrofurans substituted in the 2-position, Eur. J. Org. Chem. 23 (2005) 4929-4949; (b) F.L. Zhu, Y.H. Wang, D.Y. Zhang, et al., Enantioselective synthesis of highly functionalized dihydrofurans through copper-catalyzed asymmetric formal [3 + 2] cycloaddition of b-ketoesters with propargylic esters, Angew. Chem. Int. Ed. Engl. 53 (2014) 10223-10227; (c) M. Li, S. Lin, Z. Dong, et al., Organocatalyzed anion relay leading to functionalized 2,3-dihydrofurans, Org. Lett. 15 (2013) 3978-3981. |
| [3] | (a) T. Wang, S. Ye, Phosphine-catalyzed [3 + 2] cycloaddition of allenoates with trifluoromethylketones: synthesis of dihydrofurans and tetrahydrofurans, Org. Biomol. Chem. 9 (2011) 5260-5265; (b) H. Yu, J. Han, J. Chen, et al., Preparation of (E)-4-aryl-1,1,1-trifluoro-3-tosylbut-3-en-2-ones as fluorinated building blocks and their application in ready and highly stereoselective routes to trans-2,3-dihydrofurans substituted with trifluoromethyl and sulfonyl groups, Eur. J. Org. Chem. 16 (2012) 3142-3150. |
| [4] | (a) Z.M. Wang, X.Z. Xu, O. Kwon, Phosphine catalysis of allenes with electrophiles, Chem. Soc. Rev. 43 (2014) 2927-2940; (b) L.Y. Cui, S.H. Guo, B. Li, et al., Synthesis of cyclopentenyl and cyclohexenyl ketones via [3 + 2] and [4 + 2] annulations of 1,2-allenic ketones, Chin. Chem. Lett. 25 (2014) 55-57; (c) M.H. Mosslemina, N. Shamsa, H. Esteghamata, H. Anaraki-Ardakanib, An efficient synthesis of functionalized tetrahydro-1H-indeno[2,1-c]pyridine derivatives by a PPh3-promoted condensation reaction between acetylene esters and 1, 3-dioxo-N-aryl-2,3-dihydro-1H-indene-2-carboxamides, Chin. Chem. Lett. 24 (2013) 1095-1098. |
| [5] | (a) T. Wang, S. Ye, Diastereoselective synthesis of 6-trifluoromethyl-5,6-dihydropyrans via phosphine-catalyzed [4 + 2] annulation of alpha-benzylallenoates with ketones, Org. Lett. 12 (2010) 4168-4171; (b) L.B. Saunders, S.J. Miller, Divergent reactivity in amine-and phosphine-catalyzed C-C bond-forming reactions of allenoates with 2,2,2-trifluoroacetophenones, ACS Catal. 1 (2011) 1347-1350. |
| [6] | (a) Y. Du, X. Lu, C. Zhang, A catalytic carbon-phosphorus ylide reaction: phosphane-catalyzed annulation of allylic compounds with electron-deficient alkenes, Angew. Chem. Int. Ed. 42 (2003) 1035-1037; (b) R. Zhou, C.Z. Wang, H.B. Song, Z.J. He, Wittig olefination between phosphine, aldehyde, and allylic carbonate: a general method for stereoselective synthesis of trisubstituted 1,3-dienes with highly variable substituents, Org. Lett. 12 (2010) 976-979. |
| [7] | (a) H. Xiao, H. Duan, J. Ye, et al., Chemoselective synthesis of trifluoromethylated g-butenolide derivatives via phosphine-promoted tandem reaction of allylic carbonates and trifluoromethyl ketones, Org. Lett. 16 (2014) 5462-5465; (b) T. Wang, L.T. Shen, S. Ye, Wittig olefination of trifluoromethyl ketones and allylic carbonates mediated by phosphines synthesis 20 (2011) 3359-3363. |
| [8] | B.C.III Gerwick, S.C. Fields, P.R. Graupner, et al., Isolation and preparation of herbicidal methylidenemevalonates, Can. Pat. Appl. (2006) 2527439. |