b State Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China
In past decades,the rapid growth of coordination polymers has received much attention due to their intriguing crystal structures and valuable properties[1, 2, 3, 4, 5]. Among them,special interest has been paid to uranium-bearing materials for their rich coordination chemistry and various physical and chemical properties[6, 7, 8, 9]. Abundant uranium crystalline solid materials including uranium organic frameworks (UOFs) and uranium inorganic frameworks (UIFs) are known. For example,a large number of UOFs constructed by carboxylate ligands have been reported by Chen et al. [10, 11, 12, 13]. Recently,Sun and Albrecht-Schmitt used a variety of organic phosphonate ligands in the syntheses of uranyl phosphonates [14, 15]. In addition,O’Hare and Lii et al. have made much contribution to the isolation of UIFs involving sulfates,phosphates, silicates,germanates and even fluorides [16, 17]. In respect of uranium fluorides and oxyfluorides,most of them feature onedimensional and two-dimensional architectures,while relatively few three-dimensional structures are known. Remarkable examples are two open-framework uranium oxyfluorides (C4N2H12)U2O4F6 (MUF-1) and [N(CH3)4][(UO2)2F5] (MUF-2),that contains piperazine and tetramethylammonium as the organic templates,respectively [18, 19]. Although a great deal of uraniumcrystalline materials were synthesized in presence of organic amines,few uranium fluoride solids in which N-donor species are directly incorporated into the backbones are known due to the preference of U atom being coordinated by F and O atoms over by N atom. As a result,for most uranium fluorides,the amines serve as templates,structure directing agents or charge compensators other than coligands. Alternatively,introduction of transition metal units offers an efficient way to combine the inorganic units and organic N-donor ligands into hybrid networks. Only one such example, [(UO2)2F8(H2O)2Zn2(4,4'-bpy)2]·(4,4'-bpy) is known as a mixedmetal uranium oxyfluoride incorporating an organic ligand [20]. Thus it is a challenging task to synthesize heterometallic uranium oxyfluoride with hybrid network structures.
On the other hand,recently our group utilized bimetallic uranyl salt,Zn(UO2)(OAc)·47H2O,as both the uranium and the zinc resources,concurrently,for the successful syntheses of a series of bimetallic uranyl complexes [21]. Following this efficacious strategy, we report in this paper two examples of heterometallic uranium oxyfluorides incorporating imidazole ligands,Zn(bpi)2(UO2)2(H2O)F6 (1) and Zn(dib)(UO2)F4·0.5H2O (2) (bpi = 1-(biphenyl-4-yl)-1H-imidazole, dib = 1,4-di(1H-imidazol-1-yl)benzene). Their syntheses, crystal structure,infrared spectroscopy,UV-vis spectroscopy and emission spectroscopy are studied.
2. Experimental 2.1. Material and methodsAll chemicals were purchased commercially and used without further purification. Powder X-ray diffractions (PXRD) patterns were performed on a D8 Focus (Bruker) diffractometer with Cu-Kα radiation Field-emission (λ = 1.5405Å ,continuous,40 kV,40 mA, increment = 0.02°). The elemental analyses of C,H,and N were conducted on a Perkin-Elmer 2400 elemental analyzer. Infrared spectra were collected from single crystals of compounds 1 and 2 using a Nicolet 6700 FT-IR spectrometer with a diamond ATR objective. Energy dispersion X-ray (EDX) analysis was obtained by an FEI/Philips XL30 ESEM-FEG. The solid-state diffuse-reflectance UV/vis spectra for powder samples were recorded on a Shimadzu UV-3600 UV/vis spectrometer. The photoluminescence (PL) excitation and emission spectra were recorded with F-7000 luminescence spectrometer equipped with a Xenon lamp of 450W as an excitation light source. The photomultiplier tube voltage was 400 V,the scan speed was 1200 nm min-1,both the excitation and the emission slit width were 5.0 nm.
2.2. SynthesesThe two compoundswere synthesized by hydrothermal reaction of Zn(UO2)(OAc)4·7H2O (50mg,0.05mmol),HF (40%,25μL, 0.5mmol),bpi (20mg,0.1mmol) for 1 or dib (20 mg,0.1mmol) for 2,and deionized water (1.0mL) in a 20 mL Teflon-lined stainless steel autoclave at 180 ℃ for 3 days,then it was cooled to room temperature naturally. Yellowcrystalswere isolated,yielding 18 mg (61% based on uranium) for 1 and 20mg (64% based on uranium) for 2. Anal. Calcd. (wt%) for C30H26F6N4O5U2Zn: C,30.59; H,2.22; N, 4.76. Found: C,30.54; H,2.28; N,4.75. Anal. Calcd. (wt%) for C24H22F8N8O5U2Zn2: C,22.85; H,1.76; N,8.88. Found: C,22.89; H, 1.78; N,8.89. Powder X-ray diffraction patterns confirm the phase purity of synthesized samples (Fig. S1 in Supporting information). Energy dispersive X-ray analyses (EDX) confirm the presence of U, Zn and F in the structures,and give the Zn to U ratio of 1:2 for 1 and 1:1 for 2 (Figs. S2 and S3 in Supporting information).
2.3. X-ray crystal structure determinationSingle-crystal X-ray diffraction analyses were performed on selected suitable single crystals for title compounds. Crystallographic data were collected at 296 K on a Bruker Apex II CCD diffractometer with graphite monochromated Mo-Ka radiation (λ = 0.71073Å ). Data processing was accomplished with the SAINT program. The crystal structures were solved by direct methods and refined on F2 by full-matrix least squares using SHELXTL-97. The final refinements included anisotropic displacement parameters for all non-hydrogen atoms. All hydrogen atoms were placed by geometrical considerations with isotropic displacement parameters equal to 1.2 times those of the parent atoms and were added to the structure factor calculation. A summary of the crystallographic data for these title compounds is listed in Table 1. Selected bond distances and angles are given in Table S1 and S2 in Supporting information.
|
|
Table 1 Crystal data and structure refinement for compounds 1 and 2. |
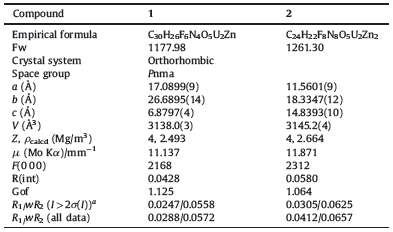
Compound 1 crystallizes in the orthorhombic space group Pnma. The asymmetric unit contains two crystallographically independent uranium sites,one zinc site,six unique fluorine sites and one bpi molecules (Fig. 1). Each uranium atom is bound to two axial oxo atoms with short uranyl bonds. The U=O bond lengths are 1.761(4) and 1.764(4)AÅ ,and the O=U=O bond angles are 177.8(2) and 179.4(2)°. In the equatorial plane,U(1) is coordinated by five bridging F atoms,forming a common UO2F5 pentagonal bipyramid. The equatorial coordination surrounding around U(2) is defined by four bridging F atom and one water molecule,thus creating a relatively rare UO2F4(H2O) pentagonal bipyramid. The U-F distances range from 2.213(3) to 2.397(3)AÅ ,while the U-O distance for the coordinated water is 2.468(4)AÅ . The Zn atom is six coordinated in a distorted octahedral environment established by two N atoms from two bpi molecules and four F atoms,which are shared with adjacent four uranyl centers. The Zn-F distances are in the range 2.000(4)-2.327(3)AÅ ,which is comparable to those in the literature [22].
|
Download:
|
| Fig. 1.ORTEP representation of the coordination environment in compound 1. Thermal ellipsoids are drawn at the 50% probability level. Symmetry codes: A,x, 0.5 - y,z; B,x,y,-1 + z; C,-0.5 + x,0.5 - y,1.5 - z. | |
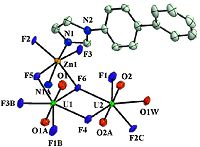
The distinct UO2F5 and UO2F4(H2O) pentagonal bipyramids share edges,forming dimers,which are in turn linked by corner sharing to construct a uranyl chain. Such chains are bridged by Zn(bpi)2 moieties through sharing edges and corners to create sheets that are parallel to [0 1 0] (Fig. 2a). Coordinated bpi molecules exit between the sheets,and hold the overall structures (Fig. 2b).
|
Download:
|
| Fig. 2.(a) Section of the inorganic layer in 1 formed by UO2F5,UO2F4(H2O) and ZnF4N2 polyhedra. (b) Overall structure of 1 viewed along c axis showing the packing of hybrid layers with coordinated bpi molecules. | |
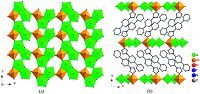
Compound 2 also crystallizes in space group Pnma,and there are two independent U(VI) sites,two unique Zn sites,eight unique F sites and one dib molecule in its asymmetric unit (Fig. 3). Both of the uranium atoms are bound to two axial oxide and five equatorial F ligands,creating UO2F5 pentagonal bipyramids that polymerize by sharing a common edge to form a dimer. The U-O lengths are 1.766(5)AÅ and 1.769(5)AÅ ,and the U-F distances range from 2.168(6)AÅ to 2.450(5)AÅ . The two Zn atoms are five coordinated by three bridging F atoms and two N atoms from two dib molecules, thereby generating trigonal bipyramidal geometries.
|
Download:
|
| Fig. 3.ORTEP representation of the coordination environment in compound 2. Thermal ellipsoids are drawn at the 50% probability level. Symmetry codes: A,x, 0.5 - y,z; B,-0.5 + x,0.5 - y,1.5 - z; C,0.5 + x,0.5 - y,2.5 - z; D,-0.5 - x,1 - y, 0.5 + z; E,-0.5 - x,1 - y,-0.5 + z. | |
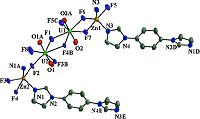
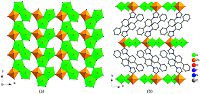
As shown in Fig. 4a,each edge-sharing dimers of UO2F5 pentagonal bipyramids share two edges and two corners with four surrounding Zn-centered polyhedra to form sheets in the ac-plane. Adjacent sheets are covalently joined by rigid dib molecules,thus creating pillared layered framework structure (Fig. 4b).
|
Download:
|
| Fig. 4.(a) Section of the inorganic layer isolated from 2 showing the connection of UO2F5 pentagonal bipyramids and ZnF3N2 trigonal bipyramids. (b) The three-dimensional structure pillared by dib molecules. | |
Compounds 1 and 2,together with the reported mixed-metal uranyl fluoride [(UO2)2F8(H2O)2Zn2(4,4'-bpy)2]·(4,4'-bpy) [20],all contain edge-sharing dimers of uranyl pentagonal bipyramidal units,which are linked by Zn-centered polyhedra in different connection modes to form the distinct neutral heterometallic zincuranium oxyfluoride inorganic layers. In 1,a six-membered ring formed by four uranyl and two zinc polyhedra is observed,while in 2 and [(UO2)2F8(H2O)2Zn2(4,4'-bpy)2]·(4,4'-bpy),four uranyl and four zinc polyhedra are arranged in different way to construct distinct eight-membered rings. It is noted that bpi with one Ndonor is covalently decorated on sides of the inorganic layers of 1. In comparison,the layers in 2 and [(UO2)2F8(H2O)2Zn2(4,4'-bpy)2]·(4,4'-bpy)are pillared by dib and 4,4'-bpy,respectively, both of which possesses two N-donors,to form three-dimensional networks. It is demonstrated that rational modulation of the Ndonor ligands can effectively control the dimensionality of resulted heterometallic uranium fluorides.
3.2. Infrared and UV-vis spectroscopyThe IR and UV-vis spectra of compounds 1 and 2 are similar, which are shown in Fig. S4 and S5 in Supporting information. The symmetric and asymmetric stretching modes of UO22+ moiety can be observed at 840 and 920 cm-1,respectively,in the IR spectra[23]. The bonds at 1060,1114,1306,1436,1540,1590,1670 and 3100 cm-1 can be attributed to the absorptions of the ring C-C and C-N stretching,ring C-H out-of-plane bending,and ring C-H stretching vibration. As shown in the UV-vis spectra,the absorptions centered at ~420 nm are ascribed to the chargetransfer transitions within the UO22+ groups. The bands at ~330 nm are probably due to the ligand-to-metal charge transfer [24].
3.3. Fluorescence spectroscopyThe emission spectra for the two title compounds are shown in Fig. 5. Several characteristic peaks ranging from 488 nm to 577 nm are present when exciting at 278 nm (the exaction spectra are depicted in Fig. S6 in Supporting information). Such spectra are typical for those uranyl-bearing materials and exhibit the characteristic vibronic structure of the UO22+ cation [25].

|
Download:
|
| Fig. 5.The emission spectra of compounds 1 and 2 excited at 278 nm. | |
In conclusion,we have synthesized and characterized two heterometallic zinc uranium oxyfluoride hybrids incorporating imidazole ligands. Both of the two compounds consist of mixedmetal zinc-uranyl fluoride inorganic layers,which are covalently linked by bpi and dib,respectively,to form the overall twodimensional and three-dimensional networks. Characteristic emissions of UO22+ moiety is demonstrated by the two uranyl fluorides. Our result reveals that structure diversities of uranium fluorides could be achieved by introducing heterometals and coligands.
AcknowledgmentsThis work was supported by National Nature Science Foundation of China (Nos. 21301168,21171162 and U1407101) and Jilin Province Youth Foundation (Nos. 20130522123JH and 20130522132JH).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.03.024.
| [1] | W. Wang, Y. Yuan, F.X. Sun, G.S. Zhu, Targeted synthesis of novel porous aromatic frameworks with selective separation of CO2/CH4 and CO2/N2, Chin. Chem. Lett. 25 (2014) 1407-1410. |
| [2] | Z.Y. Du, T.T. Xu, B. Huang, et al., Switchable guest molecular dynamics in a perovskite-like coordination polymer toward sensitive thermoresponsive dielectric materials, Angew. Chem. Int. Ed. 54 (2015) 914-918. |
| [3] | Y.X. Sun, W.Y. Sun, Influence of temperature on metal-organic frameworks, Chin. Chem. Lett. 25 (2014) 823-828. |
| [4] | Y. Peng, V. Krungleviciute, I. Eryazici, et al., Methane storage in metal-organic frameworks: current records, surprise findings, and challenges, J. Am. Chem. Soc. 135 (2013) 11887-11894. |
| [5] | T. Zheng, M. Ren, S.S. Bao, L.M. Zheng, M2(pbtcH)(phen)2(H2O)2 [M(II)≡Co, Ni]: mixed-ligated metal phosphonates based on 5-phosphonatophenyl-1,2,4-tricarboxylic acid showing double chain structures, Chin. Chem. Lett. 25 (2014) 835-838. |
| [6] | M.B. Andrews, C.L. Cahill, Uranyl bearing hybrid materials: synthesis, speciation, and solid-state structures, Chem. Rev. 113 (2013) 1121-1136. |
| [7] | T. Loiseau, I. Mihalcea, N. Henry, C. Volkringer, The crystal chemistry of uranium carboxylates, Coord. Chem. Rev. 266-267 (2014) 69-109. |
| [8] | K.X. Wang, J.S. Chen, Extended structures and physicochemical properties of uranyl-organic compounds, Acc. Chem. Res. 44 (2011) 531-540. |
| [9] | J. Qiu, P.C. Burns, Clusters of actinides with oxide, peroxide, or hydroxide bridges, Chem. Rev. 113 (2013) 1097-1120. |
| [10] | (a) Z.T. Yu, Z.L. Liao, Y.S. Jiang, et al., Construction of a microporous inorganic-organic hybrid compound with uranyl units, Chem. Commun. (2004) 1814-1815; (b) W. Chen, H.M. Yuan, J.Y. Wang, et al., Synthesis, structure, and photoelectronic effects of a uranium-zinc-organic coordination polymer containing infinite metal oxide sheets, J. Am. Chem. Soc. 125 (2003) 9266-9267. |
| [11] | (a) J. Olchowka, C. Falaise, C. Volkringer, N. Henry, T. Loiseau, Structural observations of heterometallic uranyl copper(II) carboxylates and their solid-state topotactic transformation upon dehydration, Chem. Eur. J. 19 (2013) 2012-2022; (b) C. Volkringer, N. Henry, S. Grandjean, T. Loiseau, Uranyl and/or rare-earth mellitates in extended organic-inorganic networks: a unique case of heterometallic cation-cation interaction with UVI = O-LnIII Bonding (Ln = Ce, Nd), J. Am. Chem. Soc. 134 (2012) 1275-1283; (c) J. Olchowka, C. Volkringer, N. Henry, T. Loiseau, Synthesis, structural characterization, and dehydration analysis of uranyl zinc mellitate, (UO2)Zn(H2O)4(H2-mel)·2H2O, Eur. J. Inorg. Chem. 2013 (2013) 2109-2114. |
| [12] | (a) P. Thuéry, Molecular and polymeric uranyl and thorium complexes with sulfonate-containing ligands, Eur. J. Inorg. Chem. 2014 (2014) 58-68; (b) P. Thuéry, Sulfonate complexes of actinide ions: structural diversity in uranyl complexes with 2-sulfobenzoate, Inorg. Chem. 52 (2013) 435-447. |
| [13] | (a) P.M. Cantos, L.J. Jouffret, R.E. Wilson, P.C. Burns, C.L. Cahill, Series of uranyl-4,4'-biphenyldicarboxylates and an occurrence of a cation-cation interaction: hydrothermal synthesis and in situ Raman studies, Inorg. Chem. 52 (2013) 9487-9495; (b) K.E. Knope, D.T. de Lill, C.E. Rowland, et al., Uranyl sensitization of samarium(III) luminescence in a two-dimensional coordination polymer, Inorg. Chem. 51 (2012) 201-206. |
| [14] | (a) T. Tian, W.T. Yang, H. Wang, et al., Syntheses and structures of uranyl ethylenediphosphonates: from layers to elliptical nanochannels, Inorg. Chem. 52 (2013) 7100-7106; (b) T. Tian, W.T. Yang, H. Wang, S. Dang, Z.M. Sun, Flexible diphosphonic acids for the isolation of uranyl hybrids with heterometallic UVI≡O-ZnII cation-cation interactions, Inorg. Chem. 52 (2013) 8288-8290. |
| [15] | (a) D.W. Juan, T.E. Albrecht-Schmitt, Chiral uranium phosphonates constructed from achiral units with three-dimensional frameworks, Chem. Commun. 48 (2012) 3827-3829; (b) P.O. Adelani, T.E. Albrecht-Schmitt, Differential ion exchange in elliptical uranyl diphosphonate nanotubules, Angew. Chem. Int. Ed. 49 (2010) 8909-8911; (c) A.G.D. Nelson, E.V. Alekseev, R.C. Ewing, T.E. Albrecht-Schmitt, Barium uranyl diphosphonates, J. Solid State Chem. 192 (2012) 153-160. |
| [16] | (a) M.B. Doran, B.E. Cockbain, A.J. Norquist, D. O'Hare, The effects of hydrofluoric acid addition on the hydrothermal synthesis of templated uranium sulfates, Dalton Trans. (2004) 3810-3814; (b) K. Min Ok, M.B. Doran, D. O'Hare, [(CH3)2NH(CH2)2NH(CH3)2][(UO2)2 F2(HPO4)2]: a new organically templated layered uranium phosphate fluoride -synthesis, structure, characterization, and ion-exchange reactions, Dalton Trans. (2007) 3325-3329; (c) K. Min Ok, D. O'Hare, Hydrothermal synthesis, crystal structure, and characterization of a new pseudo-two-dimensional uranyl oxyfluoride,[N(C2H5)4]2[(UO2)4(OH2)3F10], J. Solid State Chem. 180 (2007) 446-452. |
| [17] | (a) C.S. Lee, C.H. Lin, S.L. Wang, K.H. Lii, [Na7UVIO2(UVO)2(UV/VIO2)2Si4O16]: a mixed-valence uranium silicate, Angew. Chem. Int. Ed. 49 (2010) 4254-4256; (b) Q.B. Nguyen, H.K. Liu, W.J. Chang, K.H. Lii, Cs8UVI(UVIO2)3(Ge3O9)3·3H2O: a mixed-valence uranium germanate with 9-ring channels, Inorg. Chem. 50 (2011) 4241-4243. |
| [18] | P.S. Halasyamani, S.M. Walker, D. O'Hare, The first open framework actinide material (C4N2H12)U2O4F6 (MUF-1), J. Am. Chem. Soc. 121 (1999) 7415-7416. |
| [19] | K. Min Ok, M.B. Doran, D. O'Hare, [N(CH3)4][(UO2)2F5]: a new organically templated open-framework uranium oxide fluoride (MUF-2), J. Mater. Chem. 16 (2006) 3366-3368. |
| [20] | C.M. Wang, C.H. Liao, H.M. Kao, K.H. Lii, Hydrothermal synthesis and characterization of (UO2)2F8(H2O)2Zn2(4,4'-bpy)2·(4,4'-bpy), a mixed-metal uranyl aquofluoride with a pillared layer structure, Inorg. Chem. 44 (2005) 6294-6298. |
| [21] | (a) W.T. Yang, F.Y. Yi, T. Tian, W.G. Tian, S.Z. Sun, Structural variation within heterometallic uranyl hybrids based on flexible alkyldiphosphonate ligands, Cryst. Growth Des. 14 (2014) 1366-1374; (b) W.T. Yang, S. Dang, H. Wang, et al., Synthesis, structures, and properties of uranyl hybrids constructed by a variety of mono-and polycarboxylic acids, Inorg. Chem. 52 (2013) 12394-12402. |
| [22] | S.V. Matthew, H.W. Timothy, S-nitrosothiol and nitric oxide reactivity at zinc thiolates, Inorg. Chem. 48 (2009) 5605-5607. |
| [23] | A.M.S. Obbade, M. Rivenet, C. Renard, F. Abraham, [La(UO2)V2O7][(UO2)(VO4)] the first lanthanum uranyl-vanadate with structure built from two types of sheets based upon the uranophane anion-topology, J. Solid State Chem. 185 (2012) 180-186. |
| [24] | T.G. Parker, J.N. Cross, M.J. Polinski, J. Lin, T.E. Albrecht-Schmitt, Ionothermal and hydrothermal flux syntheses of five new uranyl phosphonates, Cryst. Growth Des. 14 (2014) 228-235. |
| [25] | P.O. Adelani, T.E. Albrecht-Schmitt, Syntheses of uranyl diphosphonate compounds using encapsulated cations as structure directing agents, Cryst. Growth Des. 11 (2011) 4227-4237. |