Nitrogen containing heterocycles are known subunits in many natural products and biological active compounds [1]. Among those,pyrazole is one of the important heterocycles and attracts much attention in recent years owing to its wide application in chemistry,biology,and material science as well [2, 3, 4, 5]. The synthesis of pyrazole derivatives generally involves the Knorr condensation of hydrazines with 1,3-diketones [6],multi component coupling and Claisen rearrangement [7, 8, 9, 10, 11] (Scheme 1). Recently,Wu et al. developed the copper-catalyzed tandem reaction of 2-alkynylbromobenzenes and pyrazoles that forms fused pyrazole derivatives in good yields [12]. Shortly after,Ma and coworkers developed the copper catalyzed three-component reaction of 2-alkynoates,amines and nitriles affording fully substituted pyrazoles [13]. In 2014,Yu et al. reported the regioselective pyrazole synthesis through I2-mediated oxidative C-N bond formation [14]. More recently,Allwood group found that cyclic pyrazoles can be convenient prepared from tosylhydrazones and alkynes [15]. To the best of our knowledge,the direct synthesis of pyrazole derivatives via one-step cyclization of o-alkynylchalcones and hydrazine has never been fully explored.
|
Download:
|
| Scheme 1. The representative procedure for synthesis of pyrazole derivatives. | |
In a program of developing highly efficient methodology for nitrogen-containing heterocycles [16],we recently reported the new pathway for synthesis of fused pyrazoles via cyclization of ortho-alkynylaldehydes with ketones and hydrazine under metalfree conditions [17]. As part of our continuing research,we decided to pursue the preparation of pyrazole derivatives from the easily available o-alkynylchalcones and hydrazine. Herein,we present the first efficient synthesis of fused pyrazoles via simple cyclization of o-alkynylchalcones and hydrazine under mild condition. 2. Experimental
Analytical-grade solvents were purchased,and used as received. NMR spectra were measured at 400 MHz for 1H NMR and 100 MHz for 13C NMR and calibrated from residual solvent signal. Analytical thin-layer chromatography (TLC) was performed on silica gel aluminum sheets with F-254 indicator. Visualization was accomplished by UV light. Mass spectra (MS) were measured on IonSpec 4.7 T FTMS using DART Positive. Purification by chromatography was performed using 230-400 mesh SiO2 with compressed air as a source of positive pressure (Eluent: petroleum ether/ethyl acetate = 6:1,v/v,except for compounds 2q,2r,2s and 2t,which were purified by petroleum ether/ethyl acetate = 1:1, v/v). The starting materials o-phenylethynylchalcones were prepared according to the literatures [18, 19, 20]. The purity of all final compounds was determined by HPLC analysis,confirming >95% purity. 2.1. Representative procedure for the synthesis of 2a
The mixture of o-alkynylchalcone 1a (0.2 mmol),hydrazine dihydrochloride (1.0 mmol,5 equiv.),and TEA (2.0 mmol, 10 equiv.) in methanol (2 mL) was refluxed for 12 h. Then,5 mL of water was added,extracted with ethyl acetate (3× 20 mL). Then the organic layer was dried with anhydrous sodium sulfate. Evaporation of ethyl acetate gave a yellow residue,which was further purified by gel column chromatography to afford the pure product. Some selected data were list below and others were available in Supporting information.
2a: 1H NMR (400 MHz,CDCl3): δ 7.42 (d,1H,J = 7.5 Hz),7.28 (t, 1H,J = 7.5 Hz),7.19 (dd,3H,J = 5.1,1.7 Hz),7.16-7.11 (m,1H),7.03 (dd,2H,J = 6.8,2.4 Hz),6.88 (d,1H,J = 4.0 Hz),6.08 (s,1H),5.31 (dd, 1H,J = 4.0,4.0 Hz),3.73 (dd,1H,J = 4.0,4.0 Hz),3.08 (dd,1H,J = 8.0, 8.5 Hz),2.41 (s,3H). 13C NMR (100 MHz,CDCl3): δ 153.07,145.81, 143.98,135.66,130.73,129.78,128.23,128.16,126.79,126.57, 123.87,120.23,95.90,63.32,40.07,14.50. MS ESI [M]+ m/z,260.
2b: 1H NMR (400 MHz,CDCl3): δ 7.38 (d,1H,J = 7.5 Hz),7.30- 7.24 (m,1H),7.17 (dd,3H,J = 5.0,1.8 Hz),7.13 (td,1H,J = 7.6, 1.1 Hz),7.01 (dd,2H,J = 6.5,2.9 Hz),6.88 (d,1H,J = 7.6 Hz),5.95 (s, 1H),5.30 (dd,1H,J = 4.0,4.0 Hz),3.71 (dd,1H,J = 4.0,4.0 Hz),3.08 (dd,1H,J = 8.0,8.4 Hz),2.09-2.00 (m,1H),1.00-0.93 (m,2H),0.81 (dt,2H,J = 6.2,5.0,3.7 Hz). 13C NMR (100 MHz,CDCl3): δ 159.82, 145.59,144.00,135.64,130.73,129.77,128.17,128.13,126.75, 126.54,123.82,120.12,92.58,63.33,40.04,9.99,8.12,8.07. MS ESI [M]+ m/z,286.
2c: 1H NMR (400 MHz,CDCl3): δ 7.31 (dd,1H,J = 4.0,4.6 Hz), 7.18 (t,1H,J = 7.5 Hz),7.11-7.00 (m,4H),6.98-6.86 (m,3H),6.02 (d,1H,J = 4.0 Hz),5.28 (dd,1H,J = 4.0,4.0 Hz),3.60 (td,1H,J = 16.0, 3.7 Hz),3.19 (dt,1H,J = 8.0,16.0,20.0 Hz),2.87 (m,1H),1.80-1.61 (m,2H),1.33 (dd,3H,J = 7.0,5.0 Hz),0.95 (td,3H,J = 7.4,2.3 Hz). 13C NMR (100 MHz,CDCl3): δ 162.87,145.56,143.91,135.37, 130.96,128.10,127.94,126.66,126.46,123.69,120.07,93.40, 63.21,39.80,30.60,21.08,12.17. MS ESI [M]+ m/z,289.
2d: 1H NMR (400 MHz,CDCl3): δ 8.05 (d,2H,J = 7.6 Hz),7.54 (dd,3H,J = 8.0,7.7 Hz),7.48-7.33 (m,3H),7.27 (m,4H),7.13 (s, 2H),7.05 (d,1H,J = 7.5 Hz),6.67 (s,1H),5.51 (dd,1H,J = 4.0, 3.8 Hz),3.84 (dd,1H,J = 4.0,3.4 Hz),3.33 (dd,1H,J = 8.0,8.0 Hz). 13C NMR (100 MHz,CDCl3): δ 155.97,146.50,143.97,135.53, 134.38,130.54,128.86,128.41,128.32,127.84,127.00,126.94, 125.83,123.94,120.54,93.66,63.40,40.04. MS ESI [M]+ m/z,322.
2e: 1H NMR (400 MHz,CDCl3): δ 7.92 (d,2H,J = 8.5 Hz),7.48 (d,1H,J = 7.5 Hz),7.34 (t,1H,J = 7.5 Hz),7.27-7.20 (m,4H),7.10 (dd,2H,J = 6.4,2.7 Hz),7.03 (dd,3H,J = 8.0,8.2 Hz),6.56 (s,1H), 5.47 (dd,1H,J = 4.0,4.0 Hz),3.87 (s,3H),3.81 (dd,1H,J = 4.0, 4.0 Hz),3.28 (dd,1H,J = 8.0,8.0 Hz). 13C NMR (100 MHz,CDCl3): d 159.43,155.82,146.36,143.96,135.56,130.60,129.87,128.31, 128.25,127.17,126.98,126.85,123.88,120.41,114.17,93.02, 63.66,55.35,40.03. MS ESI [M]+ m/z,352.
2f: 1H NMR (400 MHz,CDCl3): δ 8.65-8.55 (m,1H),7.99-7.91 (m,1H),7.66 (td,1H,J = 7.8,1.7 Hz),7.43 (d,1H,J = 7.5 Hz),7.24 (t, 1H,J = 7.5 Hz),7.16-7.07 (m,5H),6.97 (dd,2H,J = 6.5,2.8 Hz),6.87 (t,2H,J = 3.7 Hz),5.38 (dd,1H,J = 4.1,4.1 Hz),3.71 (dd,1H,J = 4.0, 4.2 Hz),3.13 (dd,1H,J = 8.0,8.2 Hz). 13C NMR (100 MHz,CDCl3): d 155.92,152.73,149.42,146.50,143.75,136.64,135.40,130.35, 129.77,128.38,128.27,127.01,126.86,123.87,122.39,120.59, 120.08,95.07,63.84,40.04. MS ESI [M]+ m/z,323. 3. Results and discussion
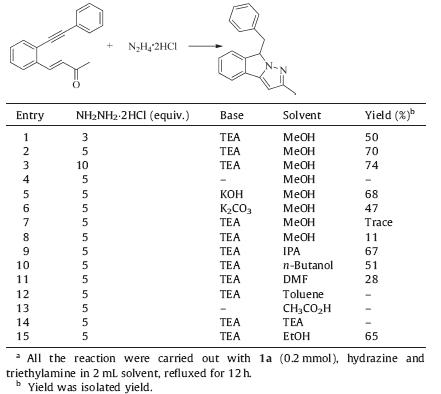
At first,we treated 2-phenylethynyl chalcone 1a with hydrazine dihydrochloride in methanol using TEA as the base at reflux for 12 h,the desired product 2a was formed in 50% yield (Table 1, entry 1). The yield was improved to 74% when 10 equiv. hydrazine was used (entry 3). Further study on the effect of base led to the observation that TEA provided the best result (entries 3-6). Solvent screening revealed that methanol was the best choice of solvent in the cyclization reaction. When ethanol was used as solvent in this reaction under same reaction condition,the desired product was obtained in 65% yield (entry 15). No reaction occurred when toluene,acetic acid and TEA were used (entries 12-14). Thus,we concluded the reaction of o-alkynyl chalcone 1a with 5 equiv. hydrazine and 10 equiv. TEA in methanol refluxing for 12 h as the standard reaction conditions.
| Table 1 Optimization of reaction conditions.a |
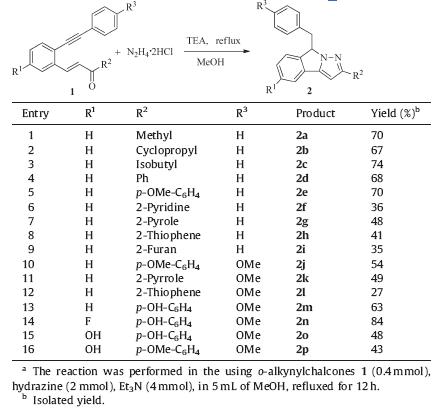
Following optimization,the scope of o-alkynylchalcones was investigated under the standard conditions and the results were summarized in Table 2. As shown in Table 2,the reaction of hydrazine with different o-alkynylchalcones in methanol afforded the corresponding pyrazoles in moderate to good yields,the substituents R1 had obvious influence on the yield of the products. For instance,the reaction of 1n having fluoro group in the phenyl ring,proceeded well and gave the product 2n in yield of 84% (entry 14). While,when 1o was used in this reaction,the product 2o was obtained in only 48% yield (entry 15). It seems that all substituents at R2 are tolerated. When alkyl and electron-rich aromatic substitutes were used as R2,reactions proceeded well and pyrazoles were obtained in 54%-84% yields (entries 1-4,5,10). In the case of heteroaromatic group was used as R2,the products were formed in lower yields (entries 6-9,11). It is worth noting that the highest yield (84%) was achieved when 3-(5-fluoro-2-((4- methoxyphenyl)ethynyl)phenyl)-1-(4-hydroxyphenyl)prop-2-en- 1-one 1n was used in this reaction (entry 14). While,the p-hydroxyphenyl substituted o-alkynyl chalcone 1m gave the 63% of 2m(entry 13). In all case,the reaction was clean,and there is no side-product was detected in this reaction.
| Table 2 Reaction of o-alkynylchalcones 1 with hydrazine in methanol.a |
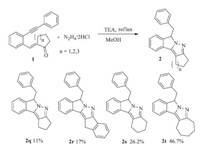
The reaction was also extended to the cyclic o-alkynylchalcones. As shown in Scheme 2,low yields of polycyclic pyrazole derivatives were observed. The 2-(2-(phenylethynyl)benzylidene) cycloheptanone 1t underwent the cyclization to afford the 2t in 47% yield. The 2-(2-(phenylethynyl)benzylidene)-cyclopentanone 1q was recovered in 50% yield along with 11% of polycyclic pyrazole 2q.
|
Download:
|
| Scheme 2. Reaction of cyclic o-alkynylchalcone with hydrazine in methanol,the reaction condition is same to that in Table 2. | |
In conclusion,we have developed the facile synthesis of pyrazole derivatives by a simple cyclization of easily accessed o-alkynylchalcones and hydrazine under mild condition. This greener synthetic methodology provides a straightforward approach to the synthesis of a variety of pyrazole derivatives under mild reaction condition.
AcknowledgmentWe are grateful to the Fundamental Research Funds for the Central Universities (No. 2042014kf0248) for support of this research.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.01.002.
| [1] | A.R. Katritzky, C.W. Rees, K.T. Potts, Comprehensive Heterocyclic Chemistry: The Structure, Reactions, Synthesis and Uses of Heterocyclic Compounds, Pergamon Press, Oxford, UK, 1984. |
| [2] | (a) J. Reedijk, Pyrazoles and imidazoles as ligands: Part I. Some simple metal (II) perchlorates and tetrafluoroborates solvated by neutral pyrazole and imidazole, Recl. Trav. Chim. Pays-Bas 88 (1969) 1451-1470;(b) J. Catalan, F. Fabero, R.M. Claramunt, et al., New ultraviolet stabilizers: 3- and 5-(2'-hydroxyphenyl)pyrazoles, J. Am. Chem. Soc. 114 (1992) 5039-5048. |
| [3] | G. Pinna, G. Loriga, P. Lazzari, et al., Tricyclic pyrazoles, Part 6. Benzofuro[3,2- c]pyrazole: a versatile architecture for CB2 selective ligands, Eur. J. Med. Chem. 82 (2014) 281-292. |
| [4] | K.O. Mohammed, Y.M. Nissan, Synthesis, molecular docking, and biological evaluation of some novel hydrazones and pyrazole derivatives as anti-inflammatory agents, Chem. Biol. Drug Des. 84 (2014) 473-488. |
| [5] | H.X. Dai, A.F. Stepan, M.S. Plummer, Y.H. Zhang, J.Q. Yu, Divergent C-H functionalizations directed by sulfonamide pharmacophores: late-stage diversification as a tool for drug discovery, J. Am. Chem. Soc. 133 (2011) 7222-7228. |
| [6] | (a) Z. Ozdemir, H.B. Kandilci, B. Gumusel, U. Calis, A.A. Bilgin, Synthesis and studies on antidepressant and anticonvulsant activities of some 3-(2-furyl)- pyrazoline derivatives, Eur. J. Med. Chem. 42 (2007) 373-379;(b) S.T. Heller, S.R. Natarajan, 1,3-Diketones from acid chlorides and ketones: a rapid and general one-pot synthesis of pyrazoles, Org. Lett. 8 (2006) 2675-2678; (c) B.A. Bhat, K.L. Dhar, S.C. Puri, et al., Synthesis and biological evaluation of chalcones and their derived pyrazoles as potential cytotoxic agents, Bioorg. Med. Chem. Lett. 15 (2005) 3177-3180. |
| [7] | (a) Y. Wang, L. Liu, L. Zhang, Combining Zn ion catalysis with homogeneous gold catalysis: an efficient annulation approach to N-protected indoles, Chem. Sci. 4 (2013) 739-746;(b) S. Cacchi, G. Fabrizi, Synthesis and functionalization of indoles through palladium-catalyzed reactions, Chem. Rev. 105 (2005) 2873-2920. |
| [8] | (a) Y.F. Wang, K.K. Toh, J.Y. Lee, S. Chiba, Synthesis of isoquinolines from α-aryl vinyl azides and internal alkynes by Rh-Cu bimetallic cooperation, Angew. Chem. Int. Ed. 50 (2011) 5927-5931;(b) P.C. Too, Y.F. Wang, S. Chiba, Rhodium(III)-catalyzed synthesis of isoquinolines from aryl ketone o-acyloxime derivatives and internal alkynes, Org. Lett. 12 (2010) 5688-5691; (c) Q. Huang, J.A. Hunter, R.C. Larock, Synthesis of substituted isoquinolines by electrophilic cyclization of iminoalkynes, J. Org. Chem. 67 (2002) 3437-3444. |
| [9] | (a) L. Zhou, Y. Shi, Q. Xiao, et al., CuBr-catalyzed coupling of N-tosylhydrazones and terminal alkynes: synthesis of benzofurans and indoles, Org. Lett. 13 (2011) 968-971;(b) I. Nakamura, Y. Mizushima, Y. Yamamoto, Synthesis of 2,3-disubstituted benzofurans by platinum-olefin-catalyzed carboalkoxylation of o-alkynylphenyl acetals, J. Am. Chem. Soc. 127 (2005) 15022-15023. |
| [10] | (a) T. Yao, R.C. Larock, Synthesis of isocoumarins and α-pyrones via electrophilic cyclization, J. Org. Chem. 68 (2003) 5936-5942;(b) R.K. Chinnagolla, M. Jeganmohan, Regioselective synthesis of isocoumarins by ruthenium-catalyzed aerobic oxidative cyclization, Chem. Commun. 48 (2012) 2030-2032. |
| [11] | X. Xu, P.Y. Zavalij, W. Hu, M.P. Doyle, Vinylogous reactivity of enol diazoacetates with donor-acceptor substituted hydrazones, synthesis of substituted pyrazole derivatives, J. Org. Chem. 78 (2013) 1583-1588. |
| [12] | X. Pan, Y. Luo, J. Wu, Route to pyrazolo[5,1-a]isoquinolines via a copper-catalyzed tandem reaction of 2-alkynylbromobenzene with pyrazole, J. Org. Chem. 78 (2013) 5756-5760. |
| [13] | B. Chen, C.Zhu,Y.Tang, S.Ma,Copper-mediatedpyrazole synthesis from2,3-allenoates or 2-alkynoates, amines and nitriles, Chem. Commun. 50 (2014) 7677-7679. |
| [14] | X. Zhang, J. Kang, P. Niu, et al., I2-mediated oxidative C-N bond formation for metal-free one-pot synthesis di,tri, and tetrasubstituted pyrazoles from α,ß unsaturated aldehydes/ketones and hydrazines, J. Org. Chem. 79 (2014) 10170-10178. |
| [15] | R.R. Merchant, D.M. Allwood, D.C. Blakemore, S.V. Ley, Regioselective preparation of saturated spirocyclic and ring-expanded fused pyrazoles, J. Org. Chem. 79 (2014) 8800-8811. |
| [16] | (a) C. Dong, L. Xie, X. Mou, Y. Zhong, W. Su, Facile synthesis of 1,3,4-benzotriazepines and 1-arylamide-1H-indazoles via palladium-catalyzed cyclization of aryl isocyanates and aryl hydrazones under microwave irradiation, Org. Biomol. Chem. 8 (2010) 4827-4830;(b) C. Dong, Z. Liao, X. Xu, H. Zhou, A new pathway for phthalazine derivatives via metal-free cyclization of ortho-alkynylphenyl ketones and hydrazine, J. Heterocycl. Chem. 51 (2014) 1282-1286. |
| [17] | J. Qiao, B. Liu, Z. Liao, One-pot to fused pyrazoles by a double cyclization of oalkynylaldehydes with ketones and hydrazine under metal-free condition, Tetrahedron 70 (2014) 3782-3787. |
| [18] | S.K. Pawar, C.D. Wang, S. Bhunia, A.M. Jadhav, R.S. Liu, Gold-catalyzed formal cycloaddition of 2-ethynylbenzyl ethers with organic oxides and α-diazoesters, Angew. Chem. Int. Ed. 52 (2013) 7559-7563. |
| [19] | X. Li, L. Li, Y. Tang, et al., Chemoselective conjugate reduction of α,β-unsaturated ketones catalyzed by rhodium amido complexes in aqueous media, J. Org. Chem. 75 (2010) 2981-2988. |
| [20] | A. Martínez, M. Fernández, J.C. Estévez, R.J. Estévez, L. Castedo, Studies on the chemistry of 2-(2-oxo-3-phenylpropyl)-benzaldehydes: novel total synthesis of 3-phenylnaphthalen-2-ols and 2-hydroxy-3-phenyl-1,4-naphthoquinones, Tetrahedron 61 (2005) 485-492. |