b College of Science, Jiangxi Agricultural University, Nanchang 330045, China
Shikonin,a naphthoquinone isolated from the Chinese herbal plant Lithospermum erythrorhizon,has attracted considerable attention due to its interesting biological activities as an antibacterial,antifungal antimicrobial,wound healing,antiinflammatory, antithrombotic,and antitumor agent [1, 2]. Besides pharmaceutical application,shikonin is also considered as a natural colorant in the printing,textile,dye,food,and cosmetic fields due to its strong and stable coloring features as well as certain antiphlogistic,antibacterial,and antiviral properties [3, 4, 5]. Hence,a simple,economical,and efficient analytical method for the qualitative and quantitative detection of shikonin is needed.
Until now,various methods have been developed for the detection of shikonin,including thin-layer chromatography chemiluminescence [6],high performance liquid chromatography [7],capillary electrophoresis (CE) [8],and electroanalysis [9]. Among them,electrochemical methods have gained considerable interest in recent years due to their simplicity,high sensitivity, good stability,low-cost instrumentation,and on-site monitoring. However,shikonin exhibits slow electron transfer at bare glass carbon electrodes,which leads to low sensitivity [10]. So,some functional materials should be synthesized to develop a sensitive electrochemical method for its detection.
Multiwalled carbon nanotubes (MWCNTs) have been extensively used in recent years due to their low cost,good mechanical strength electrical conductivity,high surface area,and their chemical stability [11]. In particular,MWCNTs can be used as a promising material for the fabrication of electrochemical sensors and biosensors mainly because they can not only improve electrochemical properties,but also provide electrocatalytic activity as well as minimize electrode surface fouling [12, 13]. Then higher sensitivities and lower detection limits can be obtained relative to traditional electrode materials. However, the intrinsic van der Waals interactions between the pristine tubes make MWCNTs bundle together on a large scale,which are insoluble in routine solvents. This limits their further application [14, 15]. Thus,it is essential to design or introduce suitable functional groups that effectively disperse MWCNTs and create enhanced functions.
Cyclodextrin (CD) is a macrocyclic glucose oligomer,consisting mostly of six,seven or eight D-glucose units forming α-,β-,or γ-CD, respectively [16, 17, 18]. CD has hydrophilic outer tails and a hydrophobic center,which makes it readily soluble in aqueous solutions and prepares it to undergo host-guest interaction with other compounds [19, 20, 21, 22]. In addition,CDs are water-soluble and environmentally friendly and can improve the dispersibility of functional materials [17, 23, 24]. Based on these,β-CD is chosen to functionalize with MWCNTs. The CD-functionalized MWCNTs composites (MWCNTs/β-CD) can simultaneously possess the properties of the individual constituent materials,such as the supramolecular recognition and enrichment capability of CD and the large surface area and high conductivity of MWCNTs,which were designed for the determination of some electroactive molecules [17, 25, 26],etc. However,the application of MWCNTs/ β-CD as an electrode material for determination of shikonin has not been reported yet.
In this paper,we demonstrated a novel electrochemical method based on a glassy carbon electrode (GCE) modified with MWCNTs/ β-CD composites. The electrochemical redox behavior of shikonin was investigated. MWCNTs/β-CD/GCE exhibited excellent enhancement effect on the electrochemical redox reactions of shikonin compared with bare GCE,β-CD/GCE and MWCNTs/ GCE,which was attributed to the synergistic effect of β-CD and MWCNTs. Consequently,a voltammetric method for shikonin was developed based on MWCNTs/β-CD/GCE and used for the detection of shikonin in urine samples with satisfactory results (Scheme 1).
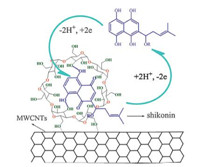
|
Download:
|
| Scheme 1. The redox mechanism of shikonin at the MWCNTs/β-CD modified electrode. | |
Chemicals and reagents: MWCNTs (purity > 95%) were purchased from Shenzhen Nanotech Port Co.,Ltd. β-CD was obtained from Aladdin Reagent Co.,Ltd. Shikonin was purchased from Biopurify. Shikonin stock solution (0.01 mol/L) prepared with absolute ethyl alcohol was stored at 278-281 K. Lithium perchlorate (LiClO4),disodium hydrogen phosphate (Na2HPO4),and sodium dihydrogen phosphate dehydrate (NaH2PO4) were obtained from Sinopharm chemical reagent Co.,Ltd. All other reagents were of analytical grade,and double distilled water was used throughout the experiment. 2.1. Apparatus
The cyclic voltammetric and electrochemical impedance spectroscopy measurements were carried out on a CHI660D electrochemical workstation (Shanghai,China). A three-electrode cell (5 mL) was used with the modified glassy carbon electrode (GCE) as the working electrode,a saturated calomel electrode (SCE) as the reference electrode and a platinum foil electrode as the counter electrode. All potentials were measured and reported vs. the SCE and all experiments were carried out at room temperature. The differential pulse voltammetry (DPV) was carried out in a potential range from 0.35 V to 0.75 V with the parameters of an increment potential of 0.004 V,a pulse amplitude of 0.05 V,a pulse width of 0.2 s,a sample width of 0.02 s,a pulse period of 0.5 s,and a quiet time of 20 s. 2.2. Preparation of different modified electrodes
Prior to modification,the GCEs were polished with chamois leather containing 0.05 μm Al2O3 slurry,rinsed thoroughly with double distilled water,then washed successively with double distilled water,anhydrous ethanol,and acetone in an ultrasonic bath,and dried under N2 before use. The β-CD suspension (1.0 wt%) was prepared by adding 10 mg β-CD powder into 1 mL deionized water. Then,1 mg MWCNTs were added into the as-prepared β-CD solution and stable MWCNTs/β-CD suspensions (1 mg/mL) were obtained by sonicating for 1 h. To obtain MWCNTs/β-CD modified GCE (MWCNTs/β-CD/GCE),5 μL dispersion was dropped onto the clean GCE surface and dried at room temperature. For the sake of comparison,MWCNTs/GCE and β-CD/ GCE were fabricated in a similar method. 3. Results and discussion 3.1. Surface morphologies of the MWCNTs and MWCNTs/β-CD films
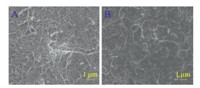
Fig. 1 shows scanning electron microscope (SEM) images of MWCNTs (A) and MWCNTs/β-CD (B). From Fig. 1A,it can be seen that the MWCNTs could not be well dispersed and they always formed badly ordered agglomerates. However,after modification with β-CD,they could be dispersed uniformly due to the van der Waals forces between MWCNTs and β-CD and the hydrogenbonding interaction between adjacent β-CD molecules [27, 28]. Meanwhile,a number of porous interspaces were also obtained from the surface morphology of MWCNTs/β-CD film,which was beneficial to maintain a large electroactive area on the electrode surface.
|
Download:
|
| Fig. 1. SEM images of MWCNTs (A) and MWCNTs/β-CD (B). | |
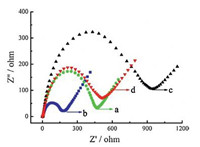
In electrochemical impedance spectroscopy measurements, the semicircle diameter of the impedance equals the electrontransfer resistance (Ret),which controls the electron-transfer kinetics of the redox probe at the electrode interface and is an important parameter. Fig. 2 presents the representative impedance spectrum of the bare GCE (a),MWCNTs/GCE (b),β-CD/GCE (c) and MWCNTs/β-CD/GCE (d) in 5.0 mmol/L K3Fe(CN)6/ K4Fe(CN)6 (1:1) containing 0.1 mol/L KCl. Compared with bare GCE (curve a),the semicircle ofMWCNTs/GCE (curve b) decreased distinctively,which was ascribed to the significantly improved electrical conductivity of MWCNTs [27, 28]. While the Rct increased dramatically at β-CD (curves c),indicating that β-CD layer hindered the electron transfer and made the interfacial charge transfer difficult. For the MWCNTs/β-CD/GCE,the semicircle of MWCNTs/β-CD/GCE was larger than that of MWCNTs/ GCE but smaller than that of β-CD/GCE,indicating β-CD on the surface of MWCNTs cannot recognize ion and block the electron transfer between electrode and Fe(CN)63-/4-. The result was consistent with that reported in literatures [23, 29].
|
Download:
|
| Fig. 2. Electrochemical impedance spectroscopy records for the bare GCE (a), MWCNTs/GCE (b),β-CD/GCE (c) and MWCNTs/β-CD/GCE (d) in 5.0 mmol/L K3Fe(CN)6/K4Fe(CN)6 (1:1) containing 0.1 mol/L KCl. | |
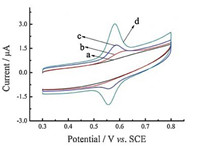
The electrochemical behavior of shikonin on bare GCE (a),β-CD/ GCE (b),MWCNTs/GCE (c),and MWCNTs/β-CD/GCE (d) electrodes was investigated by cyclic voltammetry (CV) in PBS containing 2 μmol/L shikonin (Fig. 3). It is apparent from curve (a) that the electrochemical response of the shikonin was very poor at the bare GCE. At β-CD/GCE,the electrochemical response was larger than that at bare GCE,which was attributed to the host-guest interaction between the β-CD and shikonin. In contrast,a remarkable increase in peak currents and negative shift of peak potential can be observed at MWCNTs/GCE (curve c),indicating a favorable catalytic activity of MWCNTs toward the redox reaction of shikonin. Moreover,after the attachment of β-CD on the MWCNTs,the MWCNTs/β-CD/GCE showed more stable,welldefined, and quasi-reversible redox peaks of shikonin in comparison with those at MWCNTs/GCE. The highly electrocatalytic activity of MWCNTs/β-CD film could be due to the following facts: Firstly,the MWCNTs provide a large specific surface area to increase the loading amount of shikonin. Moreover,MWCNTs could accelerate the electron transfer on the electrode surface to amplify theelectrochemical signal owning to their outstanding electric conductivity; secondly,β-CD has hydrophilic outer tails and hydrophobic centers and this structural property of β-CD makes it readily undergo host-guest interaction with other compounds to form inclusion complexes. Thus,the composite film could provide an electron-transfer microenvironment to promote the electrochemical reaction of shikonin and achieve the sensitive shikonin determination.
|
Download:
|
| Fig. 3. Cyclic voltammograms of bare GCE (a),β-CD/GCE (b),MWCNTs/GCE (c) and MWCNTs/β-CD/GCE (d) in 0.1 mol/L PBS (pH 5.0) containing 2 mmol/L shikonin. Scan rate: 50 mV/s. | |
The peak potentials and the peak currents were closely related to the pH of buffer solution. Fig. 4A displayed the effect of different pH on the response of 1 μmol/L shikonin. As can be seen,the anodic and the cathodic peaks were perfect in pH 5.0 PBS. The variations of peak current with pH of the medium were shown in Fig. 4B. When the pH increased from 3.0 to 7.0,the anodic peak moved to the negative direction,indicating that the proton had participated in the redox reaction of shikonin. The anodic peak potential was proportional to pH with the linear regression equations Epa (V) = -0.0556 pH + 0.8468 (R2 = 0.9923). The slope of the equation (55.6 mV pH) was approximately close to the theoretical value of 58.5 mV pH,indicating that the electrochemical reaction involved equal numbers of proton-transfer and electron-transfer [30]. Thus,the electrode reaction of shikonin involved two electron accompanied by two proton processes. Meanwhile,the maximum value of the redox peaks appeared at the pH value of 5.0. Therefore,pH 5.0 was selected as the optimal pH for detection in the following experiments.

|
Download:
|
| Fig. 4. (A) Cyclic voltammograms of 1 μmol/L shikonin at different pH values (3.0,4.0,5.0,6.0,7.0) in PBS at MWCNTs/β-CD modified GCE. Scan rate: 50 mV/s. (B) The influences of pH on the oxidative peak current of 1 μmol/L shikonin. Scan rate: 50 mV/s. | |
To investigate the reaction kinetics,the effect of scan rate vs. the peak current of shikonin at the MWCNTs/β-CD/GCE was shown in Fig. 5A. With the increase of the scan rate,the redox peak currents increased simultaneously,accompaniedwith an enlargement of the peak separation. Moreover,both the anodic and cathodic peak currents increased linearly with the scan rate from 25mV/s to 500 mV/s,indicating an adsorption controlled process. The regression equations were Ipa(μA) = 0.0569 v (mV/s) - 0.8752 (R2 = 0.9969) and Ipc(mA) = -0.0594 v (mV/s) + 2.0577 (R2 = 0.9975),respectively.

|
Download:
|
| Fig. 5. CVs of 2 mmol/L shikonin at MWCNTs/β-CD/GCE in 0.1 mol/L PBS (pH 5.0) at different scan rate (A); plots of anodic and cathodic peak currents against the scan rate (B) and plots of anodic and cathodic potentials against the logarithmic scan rate (C). Scan rate (mV/s): 25,50,80,100,120,150,180,200,250,300,400 and 500. Scan rate: 50 mV/s. | |
In addition,as shown in Fig. 5C,at higher scan rates,the anode peak potential (Epa) and cathode peak potential (Epc) had a linear relationship with the logarithm of scan rate (lgv). The regression equations were Epa = 0.0819lgv + 0.4167 (R2 = 0.9959) and Epc = -0.0522lgv - 0.6604 (R2 = 0.9933),respectively. According to Laviron’s equations [31]:
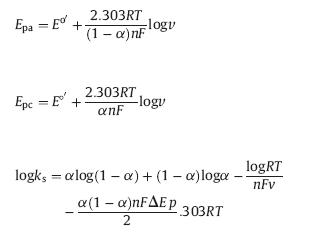
Therefore,the electron-transfer coefficient (α),electron-transfer number (n) and electrode reaction rate constant (ks)were calculated as 0.6,1.89 and 2.13 s-1,respectively. These results indicated that MWCNTs/β-CD exhibited fast electron-transfer kinetics. 3.6. Electrochemical determination of shikonin
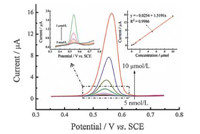
Under the optimized experimental conditions,the DPVs of shikoninwith different concentrations at the MWCNTs/β-CD/GCE were recorded (Fig. 6). The oxidation peak current of shikonin at the MWCNTs/β-CD/GCE increased linearly with the increase in the shikonin concentration from 5.0 nmol/L to 10.0 μmol/L (Inset of Fig. 6) and the corresponding linear function was Ipa = 1.5191, c - 0.0254 (R2 = 0.9986),with a detection limit of 1 nmol/L (S/N = 3). The linear range was much wider than that of the poly(diallyldimethylammoniumchloride)/graphene sheetsmodified GCE (94.72 nmol/L-3.789 μmol/L) [32],glass carbon electrode in 0.16 mol/LHAc-NaAc (20.8 nmol/L-1.82 μmol/L) [33] and PEDOT/GCE (7 nmol/L-10 μmol/L) [2]. These results thus indicated that MWCNTs/β-CD nanohybrids were excellent sensing materials for the detection of shikonin.
|
Download:
|
| Fig. 6. DPV of different concentrations of shikonin (0.005,0.01,0.05,0.1,0.5,1,2,5 and 10 mmol/L) at MWCNTs/β-CD/GCE in 0.1 mol/L PBS (pH 5.0). Inset: i vs. c shikonin plots. Pulse amplitude: 50 mV,pulse width: 0.2 s,pulse increment: 4 mV. | |
To demonstrate the reproducibility of the MWCNTs/β-CD/GCE, 1 μmol/L shikonin was determined successively 15 times using the same electrode. The value of relative standard deviation (RSD) was 4.32%,which indicated the MWCNTs/β-CD/GCE had good repeatability. For evaluating the long-term stability of the MWCNTs/b- CD/GCE,it was stored in the air at room temperature and used for monitoring 2 μmol/L shikonin daily over a period of 3 weeks. The test results showed that there was no significant change of the current responses,indicating the MWCNTs/β-CD/GCE was quite stable.
Potential interference in the determination of shikonin was studied. Under the optimized conditions,the oxidation peak current of 1.0 μmol/L shikoninwas individuallymeasured inthe presence of different concentrations of interferents. Itwas foundthat 100-fold of K+,Na+,Ni2+,Al3+,Cl-,NO3-,SO4 2-,PO43-,20-fold of glucose,folic acid,citric acid,ascorbic acid and uric acid did not interferewith the detection,with the peak current changes less than ±5%. 3.8. Sample determination
The proposed method was also applied to the determination of shikonin in spiked urine samples which were obtained from volunteers of Jiangxi Science and Technology Normal University Hospital. For sample pretreatment,the urine samples were diluted 100 times with PBS (pH 5.0) and the urine samples were further centrifuged for 5 min at 4000 rpm to remove the suspended particles. The determination of shikonin in urine samples was performed by a standard addition method. The analytical results were shown in Table 1. The recoveries were found to be in the range from 97.0% and 100.6%,indicating that the MWCNTs/β-CD/ GCE was applicable for real sample detection.
| Table 1 Recovery data for diluted (100-fold) urine samples added with different shikonin concentrations. |
In summary,a new modified electrode was successfully fabricated for the sensitive determination of shikonin based on MWCNTs/β-CD composite. MWCNTs can be well dispersed in an aqueous solution with the help of β-CD. The MWCNTs/β-CD nanomaterial combined the advantages of MWCNTs (large surface area and high conductivity) and CD (high supramolecular recognition capability). Then,the modified electrode exhibited enhanced electrocatalytic activity toward shikonin oxidation with a low detection limit. Moveover,the modified electrode showed good reproducibility and long-term stability,as well as high selectivity with no interference from other potential competing species.
AcknowledgmentsWe are grateful to the National Natural Science Foundation of China (No. 51302117),the Natural Science Foundation of Jiangxi Province (Nos. 20122BAB216011 and 20122BAB213007),Jiangxi Provincial Department of Education (No. GJJ13258),Postdoctoral Science Foundation of China (No. 2014M551857),and the Science and Technology Landing Plan of Universities in Jiangxi province (No. KJLD12081) for their financial support of this work.
| [1] | V.P. Papageorgiou, A.N. Assimopoulou, E.A. Couladouros, et al., The chemistry and biology of alkannin, shikonin, and related naphthazarin natural products, Angew. Chem. Int. Ed. 38 (1999) 270-301. |
| [2] | L.P. Wu, L.M. Lu, J.K. Xu, et al., Electrochemical determination of the anticancer herbal drug shikonin at a nanostructured poly(hydroxymethylated-3,4-ethylenedioxythiophene) modified electrode, Electroanalysis 25 (2013) 2244-2250. |
| [3] | J. Han, X.C. Weng, K.S. Bi, Antioxidants from a Chinese medicinal herb - Lithospermum erythrorhizon, Food Chem. 106 (2008) 2-10. |
| [4] | Y. Hu, Z.H. Jiang, K.S.Y. Leung, Z.Z. Zhao, Simultaneous determination of naphthoquinone derivatives in boraginaceous herbs by high-performance liquid chromatography, Anal. Chim. Acta 577 (2006) 26-31. |
| [5] | Y.I. Huang, Y.H. Cheng, C.C. Yu, T.R. Tsai, T.M. Cham, Microencapsulation of extract containing shikonin using gelatin-acacia coacervation method: a formaldehydefree approach, Colloids Surf. B 58 (2007) 290-297. |
| [6] | N. Sharma, U.K. Sharma, A.P. Gupta, et al., Simultaneous densitometric determination of shikonin, acetylshikonin, and β-acetoxyisovaleryl-shikonin in ultrasonic- assisted extracts of four Arnebia species using reversed-phase thin layer chromatography, J. Sep. Sci. 32 (2009) 3239-3245. |
| [7] | H. Yamamoto, K. Yazaki, K. Inoue, Simultaneous analysis of shikimate-derived secondary metabolites in Lithospermum erythrorhizon cell suspension cultures by high-performance liquid chromatography, J. Chromatogr. B 738 (2000) 3-15. |
| [8] | Y. Sun, T. Guo, Y. Sui, F.M. Li, Quantitative determination of rutin, quercetin, and adenosine inFlos Carthamiby capillary electrophoresis, J, Sep. Sci. 26 (2003) 1203- 1206. |
| [9] | B.R. Lichtenstein, J.F. Cerda, R.L. Koder, P. Leslie Dutton, Reversible proton coupled electron transfer in a peptide-incorporated naphthoquinone amino acid, Chem. Commun. 2 (2009) 168-170. |
| [10] | R. Chaisuksant, A. Voulgaropoulos, A.S. Mellidis, V.P. Papegeorgiou, Voltammetric determination of total alkannin using a glassy carbon electrode, Analyst 118 (1993) 179-182. |
| [11] | P.M. Ajayan, Nanotubes from carbon, Chem. Rev. 99 (1999) 1787-1800. |
| [12] | L.L. Zhang, X.S. Zhao, Carbon-based materials as supercapacitor electrodes, Chem. Soc. Rev. 38 (2009) 2520-2531. |
| [13] | C. Wei, L.M. Dai, A. Roy, T. Tia Benson, Multifunctional chemical vapor sensors of aligned carbon nanotube and polymer composites, J. Am. Chem. Soc. 128 (2006) 1412-1413. |
| [14] | K.X. Zhang, L.M. Lu, J.K. Xu, et al., Facile synthesis of the necklace-like graphene oxide-multi-walled carbon nanotube nanohybrid and its application in electrochemical sensing of azithromycin, Anal. Chim. Acta 787 (2013) 50-56. |
| [15] | Q.W. Li, J. Zhang, H. Yan, M.S. He, Z.F. Liu, Thionine-mediated chemistry of carbon nanotubes, Carbon 42 (2004) 287-291. |
| [16] | J. Zhang, J.K. Lee, Y. Wu, R.W. Murray, Photoluminescence and electronic interaction of anthracene derivatives adsorbed on sidewalls of single-walled carbon nanotubes, Nano Lett. 3 (2003) 403-407. |
| [17] | J.L. He, Y. Yang, X. Yang, et al., β-Cyclodextrin incorporated carbon nanotubemodified electrode as an electrochemical sensor for rutin, Sens. Actuators B 114 (2006) 94-100. |
| [18] | Y.J. Guo, S.J. Guo, J. Li, E.K. Wang, S.J. Dong, Cyclodextrin-graphene hybrid nanosheets as enhanced sensing platform for ultrasensitive determination of carbendazim, Talanta 84 (2011) 60-64. |
| [19] | C.M. Moraes, P. Abrami, E. dePaula, A. Braga, L. Fraceto, Study of the interaction between S(-) bupivacaine and 2-hydroxypropyl-β-cyclodextrin, Int. J. Pharm. 331 (2007) 99-106. |
| [20] | C.C. Harley, A.D. Rooney, C.B. Breslin, The selective detection of dopamine at a polypyrrole film doped with sulfonated β-cyclodextrins, Sens. Actuators B 150 (2010) 498-504. |
| [21] | J. Zhao, J.S. Jin, C.H. Wu, et al., Highly sensitive identification of cancer cells by combining the new tetrathiafulvalene derivative with a β-cyclodextrin/multiwalled carbon nanotubes modified GCE, Analyst 135 (2010) 2965-2969. |
| [22] | A. Abbaspour, A. Noori, A cyclodextrin host-guest recognition approach to an electrochemical sensor for simultaneous quantification of serotonin and dopamine, Biosens. Bioelectron. 26 (2011) 4674-4680. |
| [23] | Y.J. Guo, S.J. Guo, J.T. Ren, et al., Cyclodextrin functionalized graphene nanosheets with high supramolecular recognition capability: Synthesis and host-guest inclusion for enhanced electrochemical performance, ACS Nano 4 (2010) 4001- 4010. |
| [24] | B. Cappello, C. Carmignani, M. Iervolino, M. Immacolata La Rotonda, M. Fabrizio Saettone, Solubilization of tropicamide by hydroxypropyl-β-cyclodextrin and water-soluble polymers: in vitro/in vivo studies, Int. J. Pharm. 213 (2001) 75-81. |
| [25] | X.M. Xu, Z. Liu, X. Zhang, et al., β-Cyclodextrin functionalized mesoporous silica for electrochemical selective sensor: simultaneous determination of nitrophenol isomers, Electrochim. Acta 58 (2011) 142-149. |
| [26] | G.A. Rivas, M.D. Rubianes, M.C. Rodríguez, et al., Carbon nanotubes for electrochemical biosensing, Talanta 74 (2007) 291-307. |
| [27] | K. Liu, H. Fu, Y. Xie, et al., Assembly of β-cyclodextrins acting as molecular bricks onto multiwall carbon nanotubes, J. Phys. Chem. C 112 (2008) 951-957. |
| [28] | G. Alarcón-Angeles, B. Pérez-López, M. Palomar-Pardave, et al., Enhanced host- guest electrochemical recognition of dopamine using cyclodextrin in the presence of carbon nanotubes, Carbon 46 (2008) 898-906. |
| [29] | Y. Gao, Y. Cao, D.G. Yang, et al., Sensitivity and selectivity determination of bisphenol A using SWCNT-CD conjugate modified glassy carbon electrode, J. Hazard. Mater. 199-200 (2012) 111-118. |
| [30] | Y.C. Zhao, X.Y. Song, Q.S. Song, Z.L. Yin, A facile route to the synthesis copper oxide/reduced graphene oxide nanocomposites and electrochemical detection of catechol organic pollutant, CrystEngComm 14 (2012) 6710-6719. |
| [31] | E. Laviron, General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems, J. Electroanal. Chem. 101 (1979) 19-28. |
| [32] | J. An, J.P. Li, W.X. Chen, et al., Electrochemical study and application on shikonin at poly(diallyldimethylammoniumchloride) functionalized graphene sheets modified glass carbon electrode, Chem. Res. Chin. Univ. 29 (2013) 798- 805. |
| [33] | H.B. Zhou, J.Y. Wang, B.X. Yea, Electrochemical investigation of redox reactions of herbal drug Shikonin and its determination in pharmaceutical preparations, J. Anal. Chem. 65 (2010) 749-754. |





