b College of Science, Jiangxi Agricultural University, Nanchang 330045, China
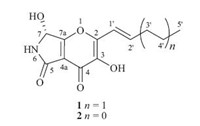
It is generally recognized that microorganisms living in unusual or specialized eco-systems may increase the chance to produce structurally unique and biologically active compounds [1]. Pyranonigrin derivatives,a kind of alkaloids with pyrano-pyrrole as core structure,were mainly isolated from the fungal species of the genus Aspergillus as well as from molds used in fermented foods such as shochu [2, 3, 4, 5]. Among these compounds,pyranonigrins A and S were reported to possess DPPH radical scavenging activity [5] and pyranonigrin A was also revealed to have suppressive effect on the expression of vascular cell adhesion molecule-1 (VCAm-1) in human umbilical vein endothelial cells (HUVECs) induced by tumor necrosis factor-a (TNF-a) [5]. The biosynthesis of pyranonigrin A was investigated by feeding experiments to show that it was composed of four units of acetate and one unit of glycine [4]. During our ongoing investigation for searching new bioactive secondary metabolites from the marine-derived fungi [6, 7, 8, 9],we have recently reported the isolation of six cytotoxic bisthiodiketopiperazine derivatives,brocazines A-F,from the culture extract of Penicillium brocae MA-231,an endophytic fungus isolated from the fresh tissue of the marine mangrove plant [9]. Further work on the remaining fraction of the fungus resulted in the isolation of a new pyranonigrin derivative,namely,pyranonigrin F (1) (Fig. 1), together with a related known compound,pyranonigrin A (2) [2, 3, 4, 5]. The structures of compounds 1 and 2 were established on the basis of spectroscopic analysis and the absolute configuration of compound 1 was established by the single-crystal X-ray diffraction. This paper describes the isolation,structure determination, stereochemical assignment,and antimicrobial activity against 12 pathogens of the isolated compounds.
|
Download:
|
| Fig. 1. Structures of compounds 1 and 2. | |
Melting points were determined with an SGW X-4 micromelting- point apparatus. Optical rotations were measured on an Optical Activity AA-55 polarimeter. CD spectra were acquired on a Chirascan spectropolarimeter. UV spectra were measured on a PuXi TU-1810 UV-visible spectrophotometer. 1D and 2D NMR spectra were recorded at 500 and 125 MHz for 1H NMR and 13C NMR,respectively,on a Bruker Avance 500 MHz spectrometer with TMS as internal standard. Mass spectra were determined on a VG Autospec 3000 or an API QSTAR Pulsar 1 mass spectrometer. Analytical and semi-preparative HPLC were performed using a Dionex HPLC system equipped with P680 pump,ASI-100 automated sample injector,and UVD340U multiple wavelength detector controlled by Chromeleon software (version 6.80). Commercial available Si gel (200-300 mesh,Qingdao Haiyang Chemical Co.),Lobar LiChroprep RP-18 (40-63 mm,Merck),and Sephadex LH-20 (Pharmacia) were used for open column chromatography. Solvents for extraction and purification were distilled prior to use. 2.2. Fungal material
The fungus P. brocae MA-231 was isolated from the fresh tissue of the marine mangrove plant Avicennia marina collected at Hainan Island of China in August 2012,as described in our previous report [9]. 2.3. Fermentation,extraction and isolation
For chemical investigations,the fresh mycelia of P. brocae MA- 231 was grown on PDA medium at 28 °C for 4 days and was then inoculated on 60 × 1 L conical flasks containing 300 mL of potatodextrose broth medium (20 g glucose,5 g peptone,and 3 g yeast extract,1 L naturally sourced and filtered seawater that was obtained from the Huiquan Gulf of the Yellow Sea near the campus of the author’s institution,pH 6.5-7.0) for 30 days at room temperature.
Thewhole fermented cultures (18 L)were filtered to separate the broth from the mycelia. The former was extracted three times with EtOAc,while the latter was extracted three times with a mixture of 80% acetone and 20% H2O. The acetone solution was evaporated under reduced pressure to afford an aqueous solution,which was then extracted three times with EtOAc. Because the TLC and HPLC profiles of the two EtOAc solutions were almost identical,theywere combined and concentrated under reduced pressure to give an extract (20.0 g),which was fractionated by silica gel vacuum liquid chromatography(VLC)using different solvents of increasingpolarity from petroleum ether (PE) to MeOH to yield 9 fractions (Frs. 1-9) based on TLC analysis. Purification of Fr. 8 (1.27 g) by column chromatography (CC) over Lobar LiChroprep RP-18 with a MeOH- H2O gradient (from 20:80 to 100:0) and then on Sephadex LH-20 (MeOH) yielded compounds 1 (33.5mg) and 2 (6.2 mg).
Pyranonigrin F (1): colorless crystals (MeOH); mp 177-179 °C; [α]D25 + 63:6 (c 0.11,MeOH); UV (MeOH) λmax (log e) 210 (4.22), 248 (3.87),312 (4.02) nm; 1H NMR and 13C NMR data,see Table 1; ESI-MS m/z 252 [M+H]+; ESI-HRMS m/z 252.0870 [M+H]+ (calcd. for C12H14NO5,252.0866).
| Table 1 1H NMR and 13C NMR spectroscopic data (500/125MHz) of compound 1. |
All crystallographic data were collected on a Bruker Smart-1000 CCD diffractometer equipped with a graphite-monochromatic Cu-Kα radiation (λ = 1.54178)Å at 293(2) K. The data were corrected for absorption by using the program SADABS [11]. The structure was solved by direct methods with the SHELXTL software package [12]. All non-hydrogen atoms were refined anisotropically. The H atoms were located by geometrical calculations,and their positions and thermal parameters were fixed during the structure refinement. The structure was refined by full-matrix least-squares techniques [13].
Crystal data for compound 1: C12H13NO5·H2O F.W. = 268.75, Triclinic,P1,unit cell dimensions α = 4.1371(3)Å ,β = 10.1027(11) Å , c = 15.4011(12)Å ,V = 632.03(10) Å 3,α = 97.233(2)8,β = 94.70108, γ = 96.0690°,Z = 2,dcalcd = 1.412 mg/m3,crystal dimensions 0.18mm× 0.13mm× 0.06 mm,m = 0.976mm-1,F(0 0 0) = 283. The 3100 measurements yielded 2369 independent reflections after equivalent data were averaged,and Lorentz and polarization corrections were applied. The final refinement gave R1 = 0.0943 and wR2 = 0.2322[I > 2σ(I)]. The Flack parameterwas 0.0(13) in the final refinement for all 3100 reflections with 2369 Friedel pairs. 3. Results and discussion
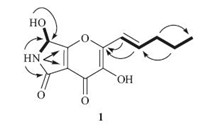
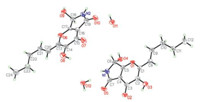
Pyranonigrin F (1) was obtained as colorless crystals and its molecular formula was determined as C12H13NO5 on the basis of positive ESI-HRMS,indicating seven degrees of unsaturation. The 1H NMR and 13C NMR data (measured in DMSO-d6) indicated the presence of one methyl,two methylenes,three methines (one oxygenated and two olefinic),and six quaternary carbons as well as three exchangeable protons (δH 6.78,8.61 and 9.70) (Table 1). Exhaustive analyses of the NMR data of 1 showed much similarity to that of pyranonigrin A (2) [2, 14]. However,signals for two methylene groups resonating at δH 1.47/2.25 (H-4'/H-3') and δC 21.5/34.6 (C-4'/C-3') were observed in the NMR spectra of 1,and the singlet methyl group for pyranonigrin A (2) was replaced by a triplet at δH 0.91 (t,7.4,H-5') in 1 (Table 1). These data,in combination with the molecular formula,indicated that the methyl group in 2 was replaced by an n-propyl group in 1. This conclusion was confirmed by the COSY cross-peaks from H-4' to H- 3' and H-5' and from H-2' to H-1' and H-3' as well as by the HMBC correlations from H-3' to C-5',from H-4' to C-2',and from H-2' to C- 2 in the HMBC spectrum (Fig. 2). Upon slowly evaporation of the solvent by storing the sample in a refrigerator,a single crystal of 1 was cultivated,making it feasible for the X-ray diffraction analysis that unequivocally confirmed the structure of 1 as deduced from the NMR spectroscopic data (Fig. 3). The Cu/Ka radiation used for the X-ray diffraction allowed the assignment of the absolute configuration of the stereogenic center in 1 as 7R.
|
Download:
|
| Fig. 2. Key HMBC (arrows) and COSY (bold lines) correlations of compound 1. | |
|
Download:
|
| Fig. 3. X-ray crystallographic structure of compound 1 (inclusive of a crystal H2O in the crystal structure. Note: a different numbering system is used for the structure in the text). | |
In addition to compound 1,a known pyranonigrin derivative, pyranonigrin A (2) [2, 3, 4, 5, 14],was also isolated and identified from the fungal strain P. brocae MA-231.
The isolated compounds 1 and 2 were evaluated for the antimicrobial activity against human and aquacultural pathogens Escherichia coli,Staphyloccocus aureus,Micrococcus luteus,Aeromonas hydrophilia,Edwardsiella tarda,Vibrio anguillarum,Vibrio harveyi, Vibrio parahaemolyticus,Alternaria brassicae,Colletotrichum gloeosprioides, Fusarium graminearum,and Gaeumannomyces graminis using the procedure as described previously [15, 16]. In the antimicrobial screening,compounds 1 and 2 displayed significant activity against S. aureus and aqua-bacteria V. harveyi and V. parahaemolyticus with MIC values of 0.5 μg/mL,which was more potent than that of the positive control chloromycetin (with MICs 8.0,2.0,and 128.0 μg/mL,respectively,for each strain). Compounds 1 and 2 also showed potent activity against plant pathogens A. brassicae and C. gloeosprioides with MICs of 0.5 μg/mL,which was also more potent than that of the positive control bleomycin (with MICs 32.0 and 4.0 μg/mL,respectively,for each strain). 4. Conclusion
One new pyranonigrin derivative,pyranonigrin F (1),was isolated from P. brocae for the first time. To our knowledge, pyranonigrin F (1) was the first example of pyranonigrin with a 1- pentenyl side chain at C-2 and the first crystal structure described for pyranonigrin derivatives. Compounds 1 and 2 showed potent activity against several human-,aqua-,and plant-pathogens.
AcknowledgmentsFinancial support from the National Natural Science Foundation of China (No. 31270403) and from the Ministry of Science and Technology of China (No. 2010CB833802) is gratefully acknowledged.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.01. 024.
| [1] | R.D. Chen, Z. Yan, J.H. Zou, N. Wang, J.G. Dai, Rubratoxin C, a new nonadride derivative from an endophytic fungus Penicillium sp. F-14, Chin. Chem. Lett. 25 (2014) 1308-1310. |
| [2] | J. Hiort, K. Maksimenka,M. Reichert, et al., New natural products from the spongederived fungus Aspergillus niger, J. Nat. Prod. 67 (2004) 1532-1543. |
| [3] | Y. Miyake, C. Ito, H. Tokuda, T. Osawa, M. Itoigawa, Evaluation of flavoglaucin, its derivatives and pyranonigrins produced by molds used in fermented foods for inhibiting tumor promotion, Biosci. Biotechnol. Biochem. 74 (2010) 1120- 1122. |
| [4] | R. Riko, H. Nakamura, K. Shindo, Studies on pyranonigrins-isolation of pyranonigrin E and biosynthetic studies on pyranonigrin A, J. Antibiot. 67 (2014) 179-181. |
| [5] | Y. Miyake, M. Muchizuki, C. Ito, M. Itoigawa, T. Osawa, Antioxidative pyranonigrins in rice mold starters and their suppressive effect on the expression of blood adhesion molecules, Biosci. Biotechnol. Biochem. 72 (2008) 1580-1585. |
| [6] | M.H. Wang, X.M. Li, C.S. Li, N.Y. Ji, B.G. Wang, Secondary metabolites from Penicillium pinophilium SD-272, a marine sediment-derived fungus, Mar. Drugs 11 (2013) 2230-2238. |
| [7] | L.H. Meng, X.M. Li, C.T. Lü, et al., Sulfur-containing cytotoxic curvularin macrolides from Penicillium sumatrense MA-92, a fungus obtained from the rhizosphere of the mangrove Lumnitzera recemosa, J. Nat. Prod. 76 (2013) 2145-2149. |
| [8] | C.Y. An, X.M. Li, H. Luo, et al., 4-Phenyl-3,4-dihydroquinolone derivatives from Aspergillus nidulans MA-143, an endophytic fungus isolated from the mangrove plant Rhizophora stylosa, J. Nat. Prod. 76 (2013) 1896-1901. |
| [9] | L.H. Meng, X.M. Li, C.T. Lü, C.G. Huang, B.G. Wang, Brocazines A-F, cytotoxic bisthiodiketopiperazine derivatives from Penicillium brocae MA-231, an endophytic fungus derived from the marine mangrove plant Avicennia marina, J. Nat. Prod. 77 (2014) 1921-1927. |
| [10] | Crystallographic data of compound 1 have been deposited in the Cambridge Crystallographic Data Centre as CCDC 1032043. The data can be obtained free of charge via http://www.ccdc.cam.ac.uk/data_request/cif (or from the CCDC, 12 Union Road, Cambridge CB21EZ, UK; fax: +44-1223-336-033; e-mail: deposit@_ccdc.cam.ac.uk). |
| [11] | G.M. Sheldrick, SADABS, Software for empirical absorption correction, University of Gottingen, Germany, 1996. |
| [12] | G.M. Sheldrick, SHELXTL, Structure determination software programs, Bruker Analytical X-ray System Inc., Wisconsin, 1997. |
| [13] | G.M. Sheldrick, SHELXL-97 and SHELXS-97, Program for X-ray crystal structure solution and refinement, University of Gottingen, Germany, 1997. |
| [14] | G. Schlingmann, T. Taniguchi, H.Y. He, et al., Reassessing the structure of pyranonigrin, J. Nat. Prod. 70 (2007) 1180-1187. |
| [15] | B.N. Meyer, N.R. Ferrigni, J.E. Putnam, et al., Brine shrimp: a convenient general bioassay for active plant constituents, Planta Med. 45 (1982) 31-34. |
| [16] | S.K.S. Al-Burtamani, M.O. Fatope, R.G. Marwash, A.K. Onifade, S.H. Al-Saidi, Chemical composition, antibacterial and antifungal activities of the essential oil of Haplophyllum tuberculatum from Oman, J. Ethnopharmacol. 96 (2005) 107-112. |





