b Department of Neonatology, Shanghai Children’s Hospital, Shanghai Jiao Tong University, Shanghai 200040, China;
c Shanghai Institute of Pharmaceutical Industry, Shanghai 200437, China
Microtubule polymerization dynamics affects many aspects of the cellular structure and function,such as cellular replication [1]. This is one of the reasons why microtubules are an attractive molecular target for anticancer agents [2]. Three small-molecule binding sites of microtubules,including the vinca,taxane,and colchicine sites,are well characterized. The colchicine site is located on the α/β-tubulin interface,and inhibitors binding to this site disrupt the polymerization of tubulins into microtubule [3, 4, 5].
Combretastatin A-4 (CA-4) binds to the colchicine site and exerts potent cytotoxicity against a wide variety of human cancer cell lines [4]. It also has the activity as vascular disrupting agents (VDAs) targeting to already established vasculature of solid tumors. CA-4 phosphate disodium (CA-4P),has displayed promising results in human cancer clinical trials,fueling continued research in novel colchicine site inhibitors (CSIs) [7, 8].
It is well known that CSIs have three important pharmacophore components,a cis-configuration linking bridge and two hydrophobic rings (rings A and B) with the appropriate dihedral angle (Fig. 1) [6, 7]. A large number of modifications of CA-4 focused on the linking bridge and B ring,respectively [6, 7, 9, 10, 11, 12, 13, 14]. There also some modifications focused on combining the linking bridge with A ring or B ring [15, 16, 17]. In our preliminary experiments,we synthesized a new CA-4 analog 22b (Fig. 1) with 1-(3,4,5- trimethoxybenzylidene)-3,4-dihydro-naphthalen-2-one skeleton, in which the relative flexible six-membered ring connects the linking bridge and B ring [18]. It showed a similar binding mode to the tubulin protein with CA-4 by molecular docking (Fig. 2A and Fig. 2B) and a strong affinity to tubulin by experiment. It has been confirmed to have strong anti-tumor activity and low toxicity in vivo.

|
Download:
|
| Fig. 1. Structures of CA-4,compounds 22b and 1. | |
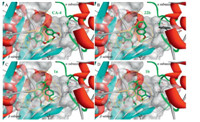
|
Download:
|
| Fig. 2. Predicted binding modes of CA-4 (A),22b (B),1a (C),and 1b (D) with the tubulin colchicine binding site. | |
In order to explore if the high biological activity still could be maintained when the connection between the six-membered ring of 22b were changed from the B ring to the A ring,the compounds 1 with 5,6,7-trimethoxy-1-benzylidene-3,4-dihydro-naphthalen-2- one skeleton were designed (Fig. 1). In addition,the B ring of this type of compounds was easy to be derived relative to the type of compound 22b. In this paper,a series of compounds with this skeleton were synthesized,and the tumor cell growth and tubulinpolymerization inhibitory activity of each analog was evaluated. Their modes of binding to tubulin were studied by molecular docking. 2. Experimental
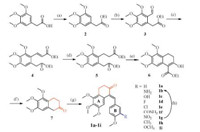
The detailed synthetic route is shown in Scheme 1. 2-(3,4,5- Trimethoxyphenyl)acetic acid was reacted with ethanol and concentrated sulfuric acid in toluene to yield intermediate compound 2 at a 99% yield. A Vilsmeier-Haack reaction with freshly prepared POCl3 and N-methylformanilide converted 2 to 3 at a 61% yield. Compound 3 was treated with (carbethoxymethylene) triphenylphosphorane in a Wittig reaction to yield compound 4 at a 92% yield. Hydration of 4 by Pd/C provided intermediate compound 5 at a 99% yield. This compound was treated with NaH in dimethoxyethane under reflux to give a Dieckmann condensation product 6 at an 88% yield,which was allowed to react with 20% HCl in ethanol to form intermediate compound 7 at a 59% yield. By the Knoevenagel reaction,7 and substituted panisaldehydes catalyzed by acetic acid and piperdine in the presence of 4Å molecular sieves afforded compound 1a-1i at 66% and 86% yields,respectively.
|
Download:
|
| Scheme 1. Reagents and conditions: (a) H2SO4,ethanol/toluene,reflux,overnight, 99%; (b) N-methylformanilide,POCl3,r.t.,40 h,61%; (c) (C6H5)3P=CHCOOEt/toluene, reflux,20 h,92%; (d) Pd/C,H2,methanol,r.t.,20 h,99%; (e) NaH/DME,reflux,30 min, 88%; (f) 20% HCl,ethanol,reflux,20 h,59%; (g) substituted p-anisaldehyde,4Å molecular sieves,acetic acid/piperidine (cat.)/CH2Cl2,r.t.,overnight,66%-86%; (h) Fe powder,acetic acid,ethanol/H2O (3:1,v/v),reflux,30 min; 89%. | |
The inhibition activities of tumor cell growth of compounds 1a-1i were evaluated against two human tumor cell lines by MTT assay [18]: The SKOV3 human ovarian cancer cell line and HT-29 human colon carcinoma cell lines. To test whether these compounds would bind to tubulin and inhibit its polymerization, compounds 1a-1c were subjected to evaluation by tubulin assembly assay [18],and 22b and CA-4 were also evaluated by this assay as positive control.
In addition,to validate observed structure-activity relationships, molecular docking studies of the compounds 22b,1a and 1b to tubulin were performed by GOLD5.0 [19]. The figures of binding modes of the inhibitors were produced by Discovery Studio 3.0 [20]. Detail methods of chemical synthesis,bioactivity evaluation, and molecular docking were showed in Supporting information. 3. Results and discussion
Nine new compounds 1a-1i (Scheme 1) with 5,6,7-trimethoxy- 1-benzylidene-3,4-dihydro-naphthalen-2-one skeleton were synthesized. All of their structures were characterized. The configuration of the target compound 1g was verified by single crystal X-ray analysis (Fig. S1 and Table S1 in Supporting information).
The inhibition activities of tumor cell growth of the target compounds were reported in Table 1. Compound 1a showed lower inhibition activity of tumor cell growth relative to 22b,which suggested that the change of the connection between the sixmembered ring from the B ring to the A ring reduced its biological activity. Like other CA-4 like tubulin polymerization inhibitors [6, 7],the substitute on the B ring has an obvious effect on biological activity. The compounds with amino,hydroxyl,cyan and fluorine substituted on position 3 showed higher inhibition activities of tumor cell growth significantly than 1a. In addition,compounds 1b even had higher activities than that of 22b and equivalent activity 22b that of CA-4,and compound 1c had higher activities than that of 22b in the HT-29 cell line. The inhibition activities of tubulin assembly of target compounds were also in Table 1. The IC50 of 1b and 1c was 3.70 and 4.60 mmol/L,equivalent to that of 22b,and the inhibition activity of 1a was found to be significantly lower. Considering that 1b and 1c,which showed strong cytotoxicity, clearly inhibited tubulin assembly,and that 1a,which showed middle cytotoxicity,showed less effect,it seems that tubulin represents a potential target for these compounds.
| Table 1 The inhibition activity of tumor cell growth and tubulin assembly of target compounds. |
The predicted binding modes of target compounds with the tubulin colchicine binding site by molecular docking were showed in Fig. 2. Compound 22b bound tightly to tubulin with a similar binding mode as CA-4 (Fig. 2A and B). The trimethoxy benzene ring bound to the hydrophobic pocket P1 in the active site,and the methoxy group substituted tetrahydronaphthalene ring bound to the hydrophobic pocket P2. Compound 1a also bound to tubulin in the same site as 22b and CA-4 (Fig. 2C),while its A rings deviated from the optimal position in the pocket P1 due to the steric hindrance of the six-membered ring. This may be why the activities of these compounds were middle. However,the substitutions on position 3 of the B ring may interact with the a subunit of tubulin to increase affinity. The amino group on the B ring of compound 1b can form hydrogen bonds with Val181 and Thr179 in a subunits (Fig. 2D),which explains its important function in improving the inhibition activity of tubulin assembly. Furthermore,other factors such as cell permeability may contribute to the high inhibition activity of tumor cell growth of compounds 1b,equivalent to that of CA-4,thought the IC50 of tubulin assembly assay of it was lower than that of CA-4 slightly. 4. Conclusion
A series of 1-(3,4,5-trimethoxy-benzylidene)-3,4-dihydronaphthalen- 2-one derivatives,in which the connection between the six-membered ring of 22b were changed from the B ring to the A ring were designed and synthesized,and their tumor cell growth and tubulin-polymerization inhibitory activity were evaluated. These compounds appear to be potential tubulin-polymerization inhibitors. Compounds 1b with amino substituted on position 3 of B ring had higher activities than that of 22b and equivalent activity to that of CA-4,and compound 1c with hydroxyl substituted on position 3 of B ring had higher activities than that of 22b in the HT-29 cell line. The binding modes of these compounds to tubulin were obtained by molecular docking,which can explain the structure-activity relationship. The studies presented here provide a new structural type for the development of novel antitumor agents.
AcknowledgmentsThe work was supported by the National Natural Science Foundation of China (Nos. 21172260 and 30901859),Shanghai Natural Science Foundation (No. 09ZR1438800) and ‘‘Chen Guang’’ Project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (No. 12CG42).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.03.022.
| [1] | M.A. Jordan, L. Wilson, Microtubules as a target for anticancer drugs, Nat. Rev. Cancer 4 (2004) 253-265. |
| [2] | C. Dumontet, M.A. Jordan, Microtubule-binding agents: a dynamic field of cancer therapeutics, Nat. Rev. Drug Discov. 9 (2010) 790-803. |
| [3] | N.H. Nam, Combretastatin A-4 analogues as antimitotic antitumor agents, Curr. Med. Chem. 10 (2003) 1697-1722. |
| [4] | L.H. Shen, H.Y. Li, H.X. Shang, et al., Synthesis and cytotoxic evaluation of new colchicine derivatives bearing 1,3,4-thiadiazole moieties, Chin. Chem. Lett. 24 (2013) 299-302. |
| [5] | T. Ai, S.Y. Shi, L.T. Chen, et al., Synthesis and anti-tumor activity evaluation of novel podophyllotoxin derivatives, Chin. Chem. Lett. 24 (2013) 37-40. |
| [6] | G.C. Tron, T. Pirali, G. Sorba, et al., Medicinal chemistry of combretastatin A4: present and future directions, J. Med. Chem. 49 (2006) 3033-3044. |
| [7] | Y.S. Shan, J. Zhang, Z. Liu, M. Wang, Y. Dong, Developments of combretastatin A-4 derivatives as anticancer agents, Curr. Med. Chem. 18 (2011) 523-538. |
| [8] | D.W. Siemann, D.J. Chaplin, P.A. Walicke, A review and update of the current status of the vasculature-disabling agent combretastatin-A4 phosphate (CA4P), Expert Opin. Invest. Drugs 18 (2009) 189-197. |
| [9] | S. Zheng, Q. Zhong, M. Mottamal, et al., Design, synthesis, and biological evaluation of novel pyridine-bridged analogues of combretastatin-A4 as anticancer agents, J. Med. Chem. 57 (2014) 3369-3381. |
| [10] | R. Romagnoli, P.G. Baraldi, C. Lopez-Cara, et al., Concise synthesis and biological evaluation of 2-aroyl-5-amino benzo [b] thiophene derivatives as a novel class of potent antimitotic agents, J. Med. Chem. 56 (2013) 9296-9309. |
| [11] | R. Álvarez, P. Puebla, J.F. Díaz, et al., Endowing indole-based tubulin inhibitors with an anchor for derivatization: highly potent 3-substituted indolephenstatins and indoleisocombretastatins, J. Med. Chem. 56 (2013) 2813-2827. |
| [12] | H.Y. Lee, J.Y. Chang, C.Y. Nien, et al., 5-Amino-2-aroylquinolines as highly potent tubulin polymerization inhibitors. Part 2. The impact of bridging groups at position C-2, J. Med. Chem. 54 (2011) 8517-8525. |
| [13] | C.H. Zheng, J. Chen, J. Liu, et al., Synthesis and biological evaluation of 1-phenyl- 1,2,3,4-dihydroisoquinoline compounds as tubulin polymerization inhibitors, Arch. Pharm. 345 (2012) 454-462. |
| [14] | Y.W. Li, J. Liu, N. Liu, et al., Imidazolone-amide bridges and their effects on tubulin polymerization in cis-locked vinylogous combretastatin-A4 analogues: synthesis and biological evaluation, Bioorg. Med. Chem. 19 (2011) 3579-3584. |
| [15] | A. Andreani, S. Burnelli, M. Granaiola, et al., Antitumor activity of substituted E- 3-(3,4,5-trimethoxybenzylidene)-1,3-dihydroindol-2-ones 1, J. Med. Chem. 49 (2006) 6922-6924. |
| [16] | J.P. Liou, Y.L. Chang, F.M. Kuo, et al., Concise synthesis and structure-activity relationships of combretastatin A-4 analogues, 1-aroylindoles and 3-aroylindoles, as novel classes of potent antitubulin agents, J. Med. Chem. 47 (2004) 4247-4257. |
| [17] | X. Ren, M. Dai, L.P. Lin, et al., Anti-angiogenic and vascular disrupting effects of C9, a new microtubule-depolymerizing agent, Br. J. Pharmacol. 156 (2009) 1228-1238. |
| [18] | J. Liu, C.H. Zheng, X.H. Ren, et al., Synthesis and biological evaluation of 1-benzylidene- 3,4-dihydronaphthalen-2-one as a new class of microtubule-targeting agents, J. Med. Chem. 55 (2012) 5720-5733. |
| [19] | GOLD 5.0., Cambridge Crystallographic Data Centre, Cambridge, UK, 2011. |
| [20] | Discovery Studio 3.0, Accelrys, Inc., San Diego, CA, 2013. |





