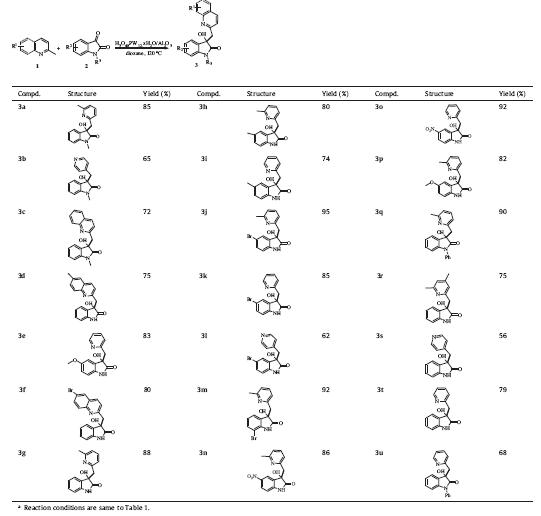
The 3-hydroxy-2-oxindole nucleus is a core structure in various biological molecules,such as donaxaridine,welwitindolinone, convolutamydine-A,dioxibrassinine,SM-130686 and maremycin- B (Fig. 1) [1]. These compounds possess extremely potent biological,chemical and pharmaceutical activities. Consequently tremendous efforts have been made to access these compounds. One of the most efficient methods for obtaining these compounds is the direct C(sp3)-H functionalization [2] of alkyl-substituted azaarene and subsequent addition to isatins. In recent years, examples catalyzed by transition metal [3],Brønsted acids [4] and microwave [5] has been reported. Although these protocols represented the most straightforward and effective manner to construct this motif,the discovery of a new,less expensive,less toxic and more environmental method is urgently needed.
|
Download:
|
| Fig. 1. Biologically active 3-hydroxy-2-oxindole derivatives. | |
Heteropoly acids have several advantages,such as readily available,Brønsted acidity,easy to handle and nontoxic,which make them attract extensive attentions in organic synthesis [6]. For example,Friedel-Crafts [7],Diels-Alder [8],esterification [9], oxidation [10] reactions catalyzed by heteropoly acids have been realized. In continuation of our interest in C(sp3)-H bond functionalization,we present herein alumina-supported heteropoly acid-catalyzed [11] reactions of 2-alkyl azaarenes with isatins to afford 3-hydroxy-2-oxindole derivatives. 2. Experimental
All major chemicals were obtained from commercial sources and used without further purification. All reactions were monitored by TLC. Column chromatography was performed on silica gel. 2.1. General procedure for the preparation of H3O40PW2·xH2O/Al2O3
Catalyst was synthesized according to the literature [11]. H3O40PW2·xH2O (2 g) was dissolved in 8 mL of methanol, then 8 g alumina was added to this solution and stirred for 4 h. The performed catalyst was dried at 80 °C for removal of methanol and subsequently calcined at 100 °C for 3 h. 2.2. General procedure for C(sp3)-H functionalization of azaarenes
A 25 mL Schlenk tube equipped with a magnetic stirring bar was charged with 2,6-lutidine (0.75 mmol,3 equiv.),1-methyl isatin (0.25 mmol),dioxane (0.5 mL) and H3O40PW2·xH2O/Al2O3 (0.03 equiv.). The tube was sealed and heated at 120 °C for 24 h. After completion of the reaction,the solution was extracted with ether (3× 10 mL). The organic layer was dried with anhydrous Na2SO4,and concentrated under vacuum. The residue was chromatographed on a silica gel column eluted with a mixture of petroleum ether and ethyl acetate (3:1) to give pure products. 3. Results and discussion
For optimization of conditions,the reaction of 2,6-lutidine (1a) with 1-methyl isatin (2a) was chosen as a model reaction. In our initial screening experiments,the effects of various aluminasupported heteropoly acids were examined. To our delight,all of the heteropoly acids catalyzed the reaction (Table 1,entries 1-3) and phosphotungstic acid (H3O40PW2·xH2O/Al2O3) afforded the desired products with the highest yield (85%). No desired product was obtained when not any catalysts was added (Table 1,entry 4). Encouraged by this result,we next investigated the effect of solvent on the reaction. When the reactions were conducted in ethylene glycol (EG),ethanol,dichloroethane,moderate to good yields of the desired products were obtained (Table 1,entries 8, 10 and 12). Other solvents such as N,N-dimethylformamide (DMF), dimethyl sulphoxide (DMSO),toluene,H2O,1,2-dimethoxyethane (DME) and THF lead to a lower yields (Table 1,entries 5-7,9, 11 and 13).
| Table 1 Optimization of heteropoly acid catalyzed C(sp3)-H bond functionalization.a |
It is worthwhile to note that when the loading of catalyst was reduced to 0.1,0.05 and even 0.03 equiv.,the yield of the product was remained unaffected (Table 1,entries 14-16). Reacting at 120 °C was essential for this reaction,either reducing or increasing the reaction temperature lead to a slightly decrease in the yield (Table 1,entries 18 and 19). Meanwhile,it was found that the yield was not proportional to the reaction time. When the reaction time was prolonged from 24 h to 48 h,the yield was almost unchanged (Table 1,entry 17).
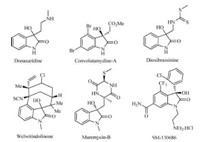
Choosing the optimized reaction condition,a series of isatins and heteroarenes were tested to examine the scope of the substrates. The results are summarized in Table 2. In general,all of the reactions are effective for affording the 3-hydroxy-2- oxindole derivatives in good to excellent yields. Istain with methyl or phenyl group on the nitrogen atom could react with various 2- substituted azaarenes with good yields (Table 2,3a-3c,3q,3u). On the other hand,isatins without methyl or phenyl substituents on the nitrogen atom were also suitable for this system. For example, 2-methylpyridine,2,6-dimethylpyridine,2,4,6-trimethylpyridine and 2-methylquinoline derivatives could react smoothly with Nunprotected istains giving 75-88% yields (Table 2,3d,3f,3g,3r,3t). Substituted istains with electron-withdrawing or electron-donating group attached to benzene rings,such as 5-nitro isatin,5- bromo istain,5-methyl istain,5-methoxyl istain and 8-bromo istain,could react smoothly with azaarene to generate the corresponding products (Table 2,3e,3h-3p). It is worthwhile to note that 4-methylpyridine was also good substrates for this reaction,although lower yields were obtained (Table 2,3b,3l,3s).
| Table 2 Synthesis of azaarene-substituted 3-hydroxy-2-oxindoles via C(sp3)-H functionalization of 2-methyl azaarenes with isatins.a |
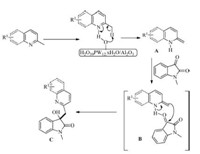
The proposed mechanism for the synthesis of 3-hydroxy-2- oxindole derivatives is described in Scheme 1. Initially,enamine A was formed with the assistance of H3O40PW2·xH2O. Then nucleophilic addition reaction between enamine A and isatins occurred to afford the desired products C via transition state B.
Finally,the recyclability of the alumina-supported heteropoly acid catalyst was investigated (Table 3). After the reaction was completed,the organic reaction solution was vacuum-filtered through a sintered glass funnel,which was washed successively with diethyl ether and dioxane. Then the catalyst can be reused directly without further purification. The recovered catalyst could be recovered and reused for six runs without loss of its activity.
|
Download:
|
| Scheme 1. Proposed mechanism of addition of C(sp3)-H bond to isatin. | |
| Table 3 Recovery and reuse of catalyst alumina-supported heteropoly acid.a |
In conclusion,an efficient system catalyzed by aluminasupported heteropoly acid has been developed for the synthesis of bioactive 3-hydroxy-2-oxindole derivatives in good to excellent yields. Several 2-methyl azaarenes and isatins could be tolerated in this reaction. The use of alumina-supported heteropoly acid permitted the product to be easily separated from the catalyst. Furthermore,the alumina-supported heteropoly acid could be recovered and recycled by a simple filtration of the reaction solution and reused for six consecutive trials without significant loss of activity. Further studies to expand this reaction to more substrates and asymmetric C(sp3)-H bond functionalization are ongoing in our lab. Acknowledgments
Financial support from the National Natural Science Foundation of China (No. 21402103),the research fund of Qingdao Agricultural University’s High-level Person (No. 631303),the Scientific Research Foundation of Shandong Province Outstanding Young Scientist Award (No. BS2013YY024) were gratefully acknowledged.
| [1] | (a) S. Peddibhotla, 3-Substituted-3-hydroxy-2-oxindole, an emerging new scaffoldfor drug discovery with potential anti-cancer and other biological activities,Curr. Bioact. Compd. 5 (2009) 20;(b) A.B. Dounay, L.E. Overman, The asymmetric intramolecular Heck reaction innatural product total synthesis, Chem. Rev. 103 (2009) 2945-3964;(c) F. Zhou, Y.L. Liu, J. Zhou, Catalytic asymmetric synthesis of oxindoles bearing atetrasubstituted stereocenter at the C-3 position, Adv. Synth. Catal. 352 (2010)1381-1407;(d) H. Hong, L.J. Huang, D.W. Teng, A spirocyclic oxindole analogue: synthesis andantitumor activities, Chin. Chem. Lett. 22 (2011) 1009-1012;(e) H. Alinezhad, A.H. Haghighi, F. Salehian, A green method for the synthesis ofbis-indolylmethanes and 3,3'-indolyloxindole derivatives using cellulose sulfuricacid under solvent-free conditions, Chin. Chem. Lett. 21 (2010) 183-186. |
| [2] | (a) Selected examples of C(sp3)-H functionalization of alkyl-substitued azaarene:J.J. Jin, H.Y. Niu, G.R. Qu, H.M. Guo, J.S. Fossey, Copper-catalysed addition of α-alkylazaarenes to ethyl glyoxylate via direct C(sp3)-H activation, RSC Adv. 2 (2012)5968-5971;(b) H. Komai, T. Yishino, S. Matsunaga, M. Kanai, Lewis acid catalyzed benzylicC H bond functionalization of azaarenes: addition to enones, Org. Lett. 13 (2011)1706-1709;(c) B. Qian, S. Guo, C. Xia, H. Huang, Lewis acid-catalyzed C-H functionalizationfor synthesis of isoindolinones and isoindolines, Adv. Synth. Catal. 2010 (352)(2010) 3195-3200;(d) B. Qian, P. Xie, Y. Xie, H. Huang, Iron-catalyzed direct alkenylation of 2-substituted azaarenes with N-sulfonyl aldimines via C-H bond activation, Org.Lett. 13 (2011) 2580-2583;(e) V.B. Graves, A. Shaikh, Lewis acid-catalyzed Csp3-H functionalization ofmethyl azaarenes with α-trifluoromethyl carbonyl compounds, Tetrahedron Lett.54 (2013) 695-698;(f) F.F. Wang, C.P. Luo, Y. Wang, G. Deng, L. Yang, Brønsted acid promoted benzylicC-H bond functionalization of azaarenes: nucleophilic addition to aldehydes, Org.Biomol. Chem. 10 (2012) 8605-8608;(g) N.N. Rao, H.M. Meshram, Microwave assisted water mediated benzylic C-Hfunctionalization of methyl aza-arenes and nucleophilic addition to aromaticaldehydes, Tetrahedron Lett. 54 (2013) 5087-5090;(h) N.N. Rao, H.M. Meshram, Microwave promoted catalyst-free benzylic C-Hfunctionalization of methylquinoline and Michael addition to beta-nitro styrene,Tetrahedron Lett. 54 (2013) 1315-1317;(i) B. Qian, S. Guo, J. Shao, et al., Palladium-catalyzed benzylic addition of 2-methyl azaarenes to N-sulfonyl aldimines via C-H bond activation, J. Am. Chem.Soc. 132 (2010) 3650-3651;(j) B.Qian, D.J. Shi, L. Yang, H. Huang,Lewis acid-catalyzed conjugate addition of sp3C-H bonds to methylenemalononitriles, Adv. Synth. Catal. 354 (2012) 2146-2150;(k) B. Qian, L. Yang, H. Huang, Cu-catalyzed direct C-H amination of 2-alkylazaareneswith azodicarboxylates via nudeophilic addition, Tetrahedron Lett. 54 (2013)711-714;(l) X.Y. Zhang, D.Q. Dong, T. Yue, S.H. Hao, Z.L. Wang, Acid ionic liquid promotedaddition of C(sp3)-H bond to aldehyde, Tetrahedron Lett. 55 (2014) 5462-5464. |
| [3] | (a) R. Niu, J. Xiao, T. Liang, X. Li, Yb(OTf)3-catalyzed addition of 2-methylazaarenes to isatins via C-H functionalization, Chin. J. Catal. 33 (2012) 1636-1641;(b) G.W. Wang, A.X. Zhou, J.J. Wang, R.B. Hu, S.D. Yang, Palladium-catalyzed sp2and sp3 C-H bond activation and addition to isatin toward 3-hydroxy-2-oxindoles,Org. Lett. 15 (2013) 5270-5273. |
| [4] | (a) R. Niu, J. Xiao, T. Liang, X. Li, Facile synthesis of azaarene-substituted3-hydroxy-2-oxindoles via Brønsted acid catalyzed sp3 C-H functionalization,Org. Lett. 14 (2012) 676-679;(b) S.V.N. Vuppalapati, Y.R. Lee, Iodine-catalyzed efficient synthesis of azaarenesubstituted 3-hydroxy-2-oxindole derivatives through sp3 H functionalization,Tetrahedron 68 (2012) 8286-8292;(c) M. Raghu, M. Rajasekhar, B.C.O. Reddy, C.S. Reddy, B.V.S. Reddy, Polyethyleneglycol (PEG-400): a mild and efficient reaction medium for one-pot synthesis of3-hydroxy-3-(pyridin-2-ylmethyl)indolin-2-ones, Tetrahedron Lett. 54 (2013)3503-3506;(d) S.A.R. Mulla, M.Y. Pathan, S.S. Chavan, A novel and efficient synthesis ofazaarene-substituted 3-hydroxy-2-oxindoles via sp3 C-H functionalization of2-methyl azaarenes and (2-azaaryl)methanes over a heterogeneous, reusablesilica-supported dodecatungstophosphoric acid catalyst, RSC Adv. 3 (2013)20281-20286. |
| [5] | H.M. Meshram, N.N. Rao, L.C. Rao, N.S. Kumar, Microwave assisted catalyst-freesynthesis of azaarene-substituted 3-hydroxy-2-oxindoles by the functionalizationof sp3 C-H bond in methyl pyridine, Tetrahedron Lett. 53 (2012) 3963-3966. |
| [6] | (a) M.M. Heravi, M.V. Fard, Z. Faghihi, Heteropoly acids-catalyzed organic reactionsin water: doubly green reactions, Green Chem. Lett. Rev. 6 (2013) 282-300;(b) H.Y. Zhang, Y.L. Dai, L. Cai, Research progress of heteropoly acid catalyzedoxidative desulfurization, Chem. Ind. Eng. Progr. 32 (2013) 809-815;(c) Y.Q. Luan, G.R. Wang, S.X. Hao, X.Q. Zhao, Y.J. Wang, The application ofheteropoly acids in condensation reaction (in Chinese), Spec. Petrochem. 27(2010) 72-77;(d) M.N. Timofeeva, Acid catalysis by heteropoly acids, Appl. Catal. A: Gen. 256(2003) 19-35;(e) S.L. Xie, Y.H. Hui, X.J. Long, C.C. Wang, Z.F. Xie, Aza-Michael addition reactionsbetween nitroolefins and benzotriazole catalyzed by MCM-41 immobilized heteropolyacids in water, Chin. Chem. Lett. 24 (2013) 28-30;(f) R. Pingaew, S. Prachayasittikul, S. Ruchirawat, V. Prachayasittikul, Tungstophosphoricacid catalyzed synthesis of N-sulfonyl-1,2,3,4-tetrahydroisoquinolineanalogs, Chin. Chem. Lett. 24 (2013) 941-944;(g) M. Rahimizadeh, T. Bazazan, A. Shiri, M. Bakavoli, H. Hassani, Preyssler-typeheteropoly acid: a new, mild and efficient catalyst for protection of carbonylcompounds, Chin. Chem. Lett. 22 (2011) 435-438;(i) Y. Zong, Y. Zhao, W. Luo, et al., Highly efficient synthesis of 2,3-dihydroquinazolin-4(1H)-ones catalyzed by heteropoly acids in water, Chin. Chem. Lett. 21(2010) 778-781. |
| [7] | (a) K. Omata, Screening of new additives to heteropoly acid catalyst for Friedel-Crafts reaction by microwave heated HTS and by Gaussian process regression,Appl. Catal. A: Gen. 407 (2011) 112-117;(b) K. Okumura, K. Yamashita, M. Hirano, M. Niwa, Active and reusable catalyst inthe Friedel-Crafts alkylation derived from a heteropoly acid, Chem. Lett. 34 (2005)716-717;(c) J. Kaur, K. Griffin, B. Harrison, I.V. Kozhevnikov, Friedel-Crafts acylationcatalysed by heteropoly acids, J. Catal. 208 (2002) 448-455. |
| [8] | D. Borkin, E. Morzhina, S. Datta, et al., Heteropoly acid-catalyzed microwaveassistedthree-component aza-Diels-Alder cyclizations: diastereoselective synthesisof potential drug candidates for Alzheimer’s disease, Org. Biomol. Chem. 9(2011) 1394-1401. |
| [9] | (a) H.T.V. Thu, H.T. Au, M.T.N. Tuyet, et al., Esterification of 2-keto-L-gulonic acidcatalyzed by a solid heteropoly acid, Catal. Sci. Technol. 3 (2013) 699-705;(b) G.D. Yadav, G. George, Single step synthesis of 4-hydroxybenzophenone viaesterification and Fries rearrangement: novelty of cesium substituted heteropolyacid supported on clay, J. Catal. A: Chem. 292 (2008) 54-57. |
| [10] | (a) J. Albert, D. Luders, A. Bosmann, D.M. Guldi, P. Wasserscheid, Spectroscopicand electrochemical characterization of heteropoly acids for their optimizedapplication in selective biomass oxidation to formic acid, Green Chem. 16(2014) 226-237;(b) E.G. Zhizhina, V.F. Odyakov, The kinetics of oxidation of 1-butene to methylethylketonein the presence of a homogeneous catalyst (complex of palladium+ Mo-V-P heteropoly acid), Chem. Eng. J. 230 (2013) 308-313. |
| [11] | Y. Li, L. Chu, M.H. Chen, L.H. Huang, S.Z. Luo, Synthesis of polytetrahydrofuran overalumina supported heteropoly acids, Acta Pet. Sin. (Pet. Process. Sect.) 22 (2006)101-105. |