b Department of Biomedical and Chemical Engineering, Syracuse University, Syracuse, NY, USA
The chlor-alkali reaction is an important basic chemical reaction,and the products,chlorine,sodium hydroxide and hydrogen are all basic raw materials. However,chlor-alkali industry consumes ten percent of the world’s electricity,and therefore an efficient gas electrode offers high potential savings in energy [1]. Currently,dimensionally stable anodes (DSAs) are mainly applied in experimental preparation,characterization and performance testing,etc.Moreover,DSAs have application in the doping coating of ruthenium-titanium oxides,in which other elements or oxides such as ZrO2,IrO2,SnO2,Ta2O5,Co3O4,or NiO are selected as the doped materials to form binary or ternary mixed oxides [2, 3, 4, 5, 6, 7, 8, 9, 10]. Variation in the content and preparation conditions of the dopant species causes differences in the composition and morphology of the coating and thereby may improve the stability of the coating and the catalytic activity of chlorine evolution. Many preparation methods,including thermal decomposition method [11],sol-gel method [12, 13],electrochemical deposition [14, 15], pulsed laser deposition [16],sputtering [17],and spray pyrolysis [18] have been considered as the main methods in coating preparation. In addition,many studies on the chlorine evolution mechanism have reported that the reaction mechanisms were all confirmed by the kinetic parameter Tafel slope and the reaction order of Cl- ; three mechanisms: the Volmer-Tafel mechanism [19],Volmer-Heyrovskey mechanism [20],and the Kristalik mechanism [21] are the most important mechanisms. In this study,based on the chlorine evolution reaction mechanism put forward by Krishtalik [21],the chlorine evolution reaction consists of two main steps: electrochemical reaction and chemical desorption reaction.

In the above reactions,Cl- is oxidized to generate adsorbed chlorine atoms (Clads) on the electrode surface,and then chemical bonding occurs between two chlorine atoms to form a molecular chlorine,which detaches from the electrode surface. The latter desorption step of the composite may be the control step since the electrode surface strongly adsorbs chlorine atoms [21]. According to this mechanism of chlorine evolution,PW91 method of the generalized density functional theory (GGA) combined with a periodic slab model has been adapted in this report. In this study, metal oxide model TinRumO2was composed of pure TiO2and RuO2 on the DNP base group,and the doping ratio of pure TiO2 and RuO2 was controlled at 3:1,1:1 and 1:3. Based on frontier molecular orbital theory,intrinsic analysis has been processed on the reaction activity caused by the electrochemical reaction and electrochemical desorption reaction from the perspective of quantum chemistry. 2. Model and method
RuO2and rutile TiO2belong to tetragonal space group P42/ MNM (symmetric D4 h),and the lattice parameters are a= 5.918 Å ,b= 6.4969 Å ,c= 29.033 3 Å ,and α=β=γ=90°.We selected the TiO2(110)andRuO2(1 1 0) surface (shown in Fig. 1a and b) for adsorption analysis. The composite oxide formed from metal oxide of Ti and Ru remains rutile,given the fact that the ionic radius of metal ion Ti4+ (0.68) is similar to that of Ru4+ (0.67). Isomorphous substitution occurs when the ion is doped,and the resulting metal oxide mixture remains as a rutile solid solution. This simplified the creation of the mixed metal oxide models using different elemental ratios. As shown in Fig. 1c-e,complex metal oxide models were conducted with different proportions of Ti and Ru: 3:1,1:1,and 1:3,using the method of isomorphous substitution to meet the high symmetry. Combined with PW91 method of generalized density functional theory (GGA),a periodic slab model has been established in DNP basis set. In theabovemodel,theatomicinnerelectronsareallreplacedwith effective nuclear potential (Effective Core Potentials referred as ECP); wave functions of the valence electrons were created by double number base and polarization function (DNP); the substrate was fixed in geometry optimization. Vacuum between two adjacent plates was fixed with a thickness of about 20 A ˚ to ensure intermolecular interactions between the plates were small enough,and all the calculations in this study were completed with Dmol3 package.

|
Download:
|
| Fig. 1. Model of Cl adsorbed on TinRumO(110). | |
Chlorine evolution is complex,since many factors may affect the chlorine evolution mechanism,mainly due to the fact that the process of chlorine evolution is an anodic process. When anodic polarization occurs at the electrode,the surface state,composition,and structure are all likely to continue changing,such as the variety of oxide films,the firm adsorption of Cl- ,and even the growth of the oxide film [21]. In addition,dissolution or passivation of the anode material may also occur,which may result in potential changes in the catalytic properties of the electrode over time. Furthermore,since the electrode preparation process is complex,it is difficult to obtain consistent,identical anodes. Further,it is difficult to measure the deposition potential, and thus it is difficult to reproduce the exact same experimental data. The above points are all the factors that influence the study of the chlorine evolution mechanism. In this report,a microscopic study has been applied to explore the mechanism of chlorine evolution with frontier orbital theory. According to frontier orbital theory,the front orbitals,the HOMO (highest occupied molecular orbital) and the LUMO (lowest unoccupied molecular orbital),have the priority function in the molecular orbital interaction. HOMO energy values reflect the ability to lose electrons. According to Koopmanns,negative HOMO level represents the first ionization energy of the material. The lower the ionization energy is,the higher the HOMO energy level would be,and the substance loses electrons more easily. The value of LUMO energy level is similar to that of molecule affinity. The lower the LUMO energy level value is,the more easily the substance receives electrons. Superposition occurs between the HOMO and the LUMO of two molecules,which are close to each other. Electrons transfer from the HOMO of one molecule to the LUMO of another molecule; therefore,the size of the energy gap difference (ΔE) between LUMO and HOMO reflects the ability of molecules to react in a certain degree,which means that the greater its value is, the more difficult it is to stimulate the molecule,and the lower the activity would be.
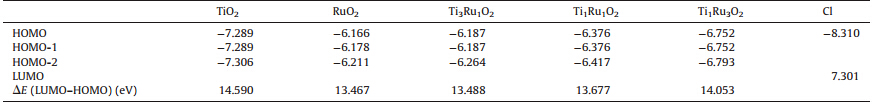
Shown in Table 1 are the calculation results based on the TinRumO2(1 1 0) model. HOMO represents the HOMO energy of the TinRumO2(1 1 0) system. And LUMO represents the LUMO energy of Cl.DEis the energy difference between the LUMO orbital of Cl and the HOMO orbital of TinRumO2 (1 1 0),characterizing the reactivity TinRumO2(1 1 0) and Cl in reaction (1).
| Table 1 Calculation results of TinRumO(1 1 0). |
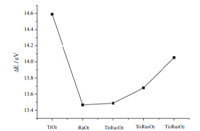
According to the data in Table 1,variation inDEof TinRumO2 (1 1 0) in reaction (1) was plotted in Fig. 2,which presents a maximumDEin pure TiO2and a minimum one in pure RuO2. The doped one with a ratio of 3:1 in Ti:Ru has the minimumDE, 13.488 eV. This is due to improvements in the catalytic activity of the system with Ru doping,which effectively reduces the energy difference between the LUMO of Cl and the HOMO of TinRumO2 (1 1 0),making reaction (1) more likely.
|
Download:
|
| Fig. 2. Variation in ΔE of reaction (1) | |
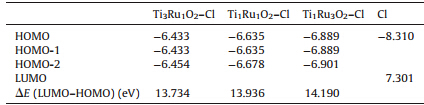
According to the electrochemical desorption mechanism, reaction (2) is the rate-limiting step of the chlorine evolution process. Listed in Table 2 are the calculation results of the Cl absorption on TinRumO2 (1 1 0) surface. HOMO represents the HOMO energy of TinRumO2 (1 1 0)-Cl system,and the value of HOMO energy reflects the affinity for losing electrons in the TinRumO2(1 1 0)-Cl system. LUMO represents the LUMO energy of Cl orbital,and the value of LUMO energy levels reflects the electronegativity Cl. In addition,DE is the energy difference between the LUMO of Cl and the HOMO of TinRumO2(1 1 0)-Cl,and the value ofDE(the energy gap) reflects the likeliness of electron transfer from the HOMO of TinRumO2(1 1 0)-Cl to the LUMO of Cl.
| Table 2 Calculation results of TinRumO(1 1 0) on the top of Ru in TinRumO(1 1 0) with Cl absorption. |
Fig. 3 plots the data in Table 2. The minimum value ofDEin the doped system with a Ti:Ru ratio of 3:1 is 13.734 eV; with a Ti:Ru ratio of 1:1 the value is 13.936 eV; and with a Ti:Ru ratio of 1:3 there is the highestDEvalue,14.190 eV. The above results indicate that Ru changes the catalytic activity; however,increased doping ratios make reaction (2) more unfavorable,reducing catalytic activity. As shown in Fig. 3,the system with a doped Ti:Ru ratio of 3:1 significantly increases the activity in reaction (2) and has the highest chlorine evolution activity.

|
Download:
|
| Fig. 3. Variation in ΔE of reaction (2) | |
Based on the combination of reaction (1),reaction (2) and the calculation results of Cl absorption on TinRumO2(1 1 0) surface, analysis is presented here. In the analysis,adsorption energy was calculated according to DEads=Eoverall-(Eadsorbat+Esurface). The larger the adsorption absolute value is; the higher the adsorption is,the more difficult desorption is. According to the d band center theory,the d-band energy center of the transition metal surface is the chemical reactivity descriptor and correlates with the adsorption energy. When the d band center is closer to the Fermi level,the effect is stronger.
As listed in Table 3,the adsorption energy of Cl at Ti31Ru3O2 (1 1 0) surface is-2.514 eV,which is much smaller than those in pure TiO2,RuO2,Ti1Ru1O2,orTi1Ru3O2. This theoretically confirms that the system with a Ti:Ru ratio of 3:1 has the highest tested catalytic activity in chlorine evolution. The system with a Ti:Ru ratio of 3:1 has a corresponding minimum value of the d-band center (-3.172 eV),which is farthest away from the Fermi level. This indicates that Cl has the minimum interaction with Ti31Ru3O2 (1 1 0) surface,so reaction (2) is prone to occur,and desorption is easier to occur,which is consistent with the adsorption energy analysis. Based on the changes in the corresponding bond length, the bond length of Cl adsorbed on Ti31Ru3O2 (1 1 0) surface is 2.283 Å . The longer the bond length is,the weaker bonding will be, and thus the smaller the binding energy will be. The more easily the bond is broken,the more likely desorption is. This is consistent with the analysis of adsorption energy and d-band energy center.
| Table 3 Variation in absorption parameter and bond length of Cl on the surfaces of TiO2, RuO2and TinRumO(1 1 0) |
Combined with studies on the chlorine evolution mechanism, microscopic analysis has been applied to the study of the electrochemical reaction [reaction (1)] and the Cl electrochemical desorption reaction [reaction (2)]. According to the adsorption strength analysis on reactions,reaction (1) is more prone to occur in the system with a doped Ti:Ru ratio of 3:1 to generate M-Cl ads intermediates,and the minimum value of ΔE is 13.488 eV. Moreover,reaction (2) is more prone to occur in the system with the doped Ti:Ru ratio of 3:1 with the precipitation of Cl2,and the minimum value of ΔEis 13.734 eV. In addition,binding energy of Cl on the Ti31Ru3O2 (1 1 0) surface of the above system has the minimum value (Ea is -2.514 eV) and reaction (2) is most favorable. Consequently,if the mechanism occurs in accordance with the chlorine evolution scheme of reactions (1) and (2),the system with the doped Ti:Ru ratio of 3:1 will have the highest catalytic activity on chlorine evolution.
AcknowledgmentsWe are grateful to the Natural Science Foundation of China (No. 51072239) and the Fundamental Research Funds for the Central Universities (No. CQDXWL-2012-032) for financial support
| [1] | S. Trasatti, Electrocatalysis in the anodic evolution of oxygen and chlorine, Electrochim. Acta 29 (1984) 1503-1512. |
| [2] | M.V. Makarova, J. Jirkovský,M. Klementová, et al., The electrocatalytic behavior of Ru0.8Co0.2OM2-x - the effect of particle shape and surface composition, Electrochim. Acta 53 (2008) 2656-2664. |
| [3] | Y.V. Pleskov, M.D. Krotova, V.I. Polyakov, et al., Electrochemical properties of amorphous nitrogen-containing hydrogenated diamondlike-carbon films, Russ. J. Electrochem. 36 (2008) 1008-1013. |
| [4] | K. Endo, Y. Katayama, T. Miura, T. Kishi, Composition dependence of the oxygenevolution reaction rate on IrxTi1 xO2 mixed-oxide electrodes, J. Appl. Electrochem. 32 (2002) 173-178. |
| [5] | J. Ribeiro, P.D.P. Alves, A.R. de Andrade, Effect of the preparation methodology on some physical and electrochemical properties of Ti/IrxSn(1-x)O2 materials, J. Mater. Sci. 42 (2007) 9293-9299. |
| [6] | F.H. Oliveira, M.E. Osugi, F.M.M. Paschoal, et al., Electrochemical oxidation of an acid dye by active chlorine generated using Ti/Sn(1-x)O2 electrodes, J. Appl. Electrochem. 37 (2007) 583-592. |
| [7] | V.G. Lourdes, H. Erzsébet, K. János, R. Ákos, D.B. Achille, Investigation of IrO2/SnO2 thin film evolution from aqueous media, Appl. Surf. Sci. 253 (2006) 1178-1184. |
| [8] | K. Macounova, M. Makarova, J. Jirkovsky, J. Franc, P. Krtil, Parallel oxygen and chlorine evolution on Ru1-xNixO2-y nanostructured electrodes, Electrochim. Acta 53 (2008) 6126-6134. |
| [9] | V.G. Lourdes, F. Sergio, D.B. Achille, Preparation and characterization of RuO2- IrO2-SnO2 ternary mixtures for advanced electrochemical technology, Appl. Catal. B: Environ. 67 (2006) 34-40. |
| [10] | J.L. Fernández, M.R. Gennero De Chialvo, A.C. Chialvo, Preparation and electrochemical characterization of TiRuxMn1-xO2 electrodes, J. Appl. Electrochem. 32 (2002) 513-520. |
| [11] | J.L. Fernández, M.R. Gennero De Chialvo, A.C. Chialvo, Ruthenium dioxide films on titanium wire electrodes by spray pyrolysis: preparation and electrochemical characterization, J. Appl. Electrochem. 27 (1997) 1323-1327. |
| [12] | M. Aparicio, L.C. Klein, Thin and thick RuO2-TiO2 coatings on titanium substrates by the sol-gel process, J. Sol-Gel Sci. Technol. 29 (2004) 81-88. |
| [13] | L. Armelao, D. Barreca, B. Moraru, A molecular approach to RuO2-based thin films: sol-gel synthesis and characterisation, J. Non-Cryst. Solids 316 (2003) 364-371. |
| [14] | M. Metikoš-Hukvić, R. Babić, F. Jović, Z. Grubač, Anodically formed oxide films and oxygen reduction on electrodeposited ruthenium in acid solution, Electrochim. Acta 51 (2006) 1157-1164. |
| [15] | V. Briss, R. Myers, H. Angerstein-Kozlowska, B.E. Conway, Electron microscopy study of formation of thick oxide films on Ir and Ru electrodes, J. Electrochem. Soc. 131 (1984) 1502-1510. |
| [16] | M. Hiratani, Y. Matsui, K. Imagawa, S. Kimura, Growth of RuO2 thin films by pulsed-laser deposition, Thin Solid Films 366 (2000) 102-106. |
| [17] | R. Kö tz, S. Stucki, Stabilization of RuO2 by IrO2 for anodic oxygen evolution in acid media, Electrochim. Acta 31 (1986) 1311-1316. |
| [18] | J. Kawakita, M. Stratmann, A.W. Hasselb, High voltage pulse anodization of a NiTi shape memory alloy, J. Electrochem. Soc. 154 (2007) C294-C298. |
| [19] | S. Trasatti, W.E.O. Grady, Mechanism of chlorine evolution on oxide anodes study of pH effects, J. Electroanal. Chem. Interfac. Electrochem. 228 (1987) 393-406. |
| [20] | S. Trasatti, G. Lodi, Electrodes of Conductive Metallic Oxides, Elsevier, New York, 1980, pp. 301-358. |
| [21] | T. Hepel, F.H. Pollak, W.E. O’Grady, Chlorine evolution and reduction processes at oriented single-crystal RuO2 electrodes, J. Electrochem. Soc. 133 (1986) 69-75. |