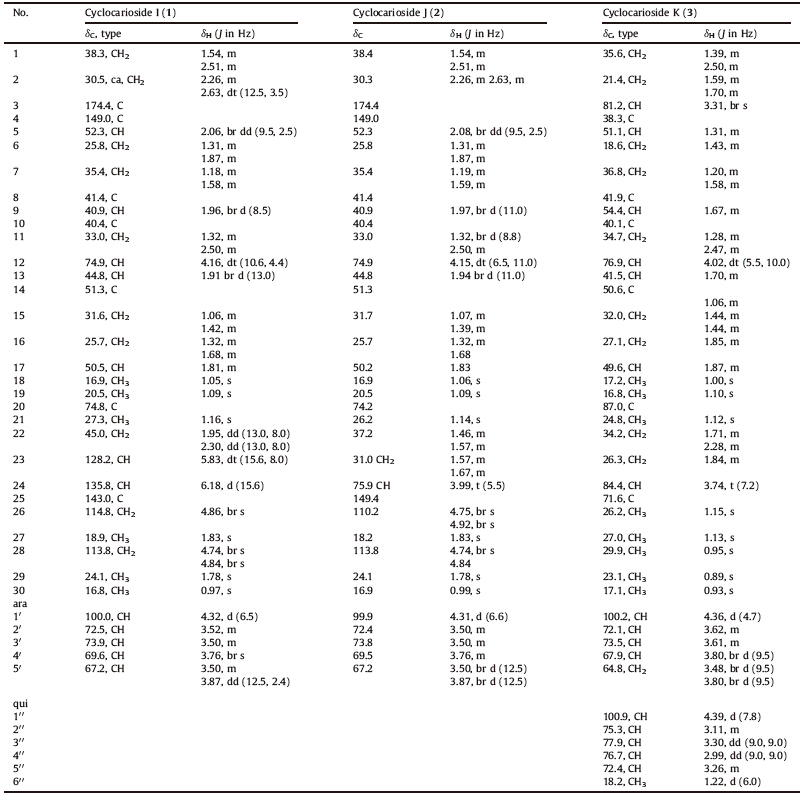
As a part of our program to assess the chemical and biological diversity of several cultivated traditional Chinese medicines,we found Chinese endemic plant,Cyclocarya paliurus (Batal) Iljinsk (Juglandaceae) is used as sweet tea for the treatment of hypertension and diabetes [1, 2] and is known to have a hypolipemic effect [3]. Previously,some dammarane-,oleanane-, and ursane-type triterpenoids,flavonoids,steroids and other compounds had been reported from C. paliurus [4, 5, 6, 7, 8, 9, 10, 11, 12, 13]. Phytochemical investigation of its leaves was undertaken to assess the chemical and biological diversity. In our previous work,3,4-secodammarane triterpenoids,epoxydammarane triterpenoid saponin,oleanane- and ursane-type triterpenoids, terpenoids,flavonoid glycoside were isolated from the ethyl acetate soluble fraction of ethanol extracts of the leaves [14, 15]. Further chemical investigation of this fraction for the purpose of identifying more novel constituents led to the isolation of two new secodammarane triterpenoid saponins (1 and 2) and a new epoxydammarane triterpenoid saponin (3) (Fig. 1). Structural determinations of these compounds were carried out mainly by spectral analysis.
|
Download:
|
| Fig 1. The structure of compounds 1-3. | |
Optical rotations were measured on PE Model 343 polarimeter. 1D- and 2D NMR spectra were obtained on an Inova 500 MHz spectrometer in acetone-d6,with solvent peaks as references. Mass spectra were obtained on a Mass Agilent 1100 Series LC-MSD-Trap- SL spectrometer (ESI-MS) and 6210 ESI-TOF spectrometer (HR-ESIMS). High-performance liquid chromatography (HPLC) preparation was performed on a Lab Alliance Prep-100 pump equipped with a Lab Alliance Model-201 UV-vis detector,and a semipreparative reversed-phase column (Grace,Allsphere ODS-25 mm, 250 × 10 mm). Column chromatography (CC) was performed on silica gel (200-300 mesh,Qingdao Marine Chemical Co.,Ltd.),RP- 18 (45-75 μm,Alltech Bulk Higt Capacity C18),and Sephadex LH- 20 (GE Healthcare,Uppsala). Thin layer chromatography (TLC) was performed on silica gel plates (GF254,Yantai Chemical Industry Research Insititute) and RP-18F254 plates (Merck). Solvents of analytical grade and chromatographic grade were purchased from Beijing Chemical Corporation. Arabinose and quinovose were from Alfa Aesar A Johnson Matthey Company. Fractions were monitored by TLC,and spots were visualized on precoated silica gel plates by spraying 10% H2SO4 in EtOH followed by heating. 2.2. Plant material
Theleaves ofC. paliuruswerecollected at Qimen,Anhui Province, China,in September 2001,and were identified by Mr. Ma Lin (Institute of Materia Medica,ChineseAcademyof Medical Sciences). A voucher specimen (No. ZH02001) has been deposited at the Herbarium of the Department of Medicinal Plants,Institute of Materia Medica,Chinese Academy of Medical Sciences. 2.3. Extraction and isolation
The air-dried and powdered leaves of C. paliurus (1 kg) were extracted with 95% ethanol (6 L × 1 h × 3) by ultrasonication (230 W,35 kHz) at room temperature. The extracts were combined and concentrated under reduced pressure (at < 60 ℃) to give a dark brown residue (51 g). The residue was suspended in water (3 L) and then partitioned with ethyl acetate (3 L × 4,29 g). The ethyl acetate fraction (28 g) was subjected to column chromatography (CC) [silica gel,500 g,column,6 × 70 cm] eluted with a gradient of increasing methanol (0-20%) in chloroform and methanol to yield fourteen fractions (A-N) on the basis of TLC analyses. Fraction M (3.9 g) was purified by CC [Sephadex LH-20 (100 g),column, 2.5 × 75 cm] eluted with petroleum ether-CHCl3-MeOH (5:5:1, 700 mL) to yield six fractions (M1-M6) on the basis of TLC analyses. The subfraction M1 (520 mg) was purified by CC [Sephadex LH-20 (50 g),column,1.5 × 75 cm] eluted with petroleum ether-CHCl3-MeOH (5:5:1,400 mL) and then applied to preparative HPLC [column,RP-18 (250 × 10 mm,5 μm); MeOH- H2O (85:15),2 mL/min flow rate,210 nm] to obtain 1 (12 mg). The subfraction M2 (1.27 g) was purified by CC [silica gel,50 g,column, 2.5 × 20 cm] eluted with a gradient of increasing methanol (10%- 15%) in chloroform and methanol to yield four fractions (M2A- M2D). The subfraction M2B (800 mg) was purified by CC [silica gel, column,2 × 25 cm] eluted with CHCl3-MeOH (95:5,1000 mL) and then applied to preparative HPLC [column,RP-18 (250 × 10 mm, 5 μm); MeOH-H2O (85:15),2 mL/min flow rate,210 nm] to obtain 2 (5 mg). The subfraction M2C (200 mg) was purified by preparative HPLC [column,RP-18 (250 × 10 mm,5 μm); MeOH- H2O (80:20),2 mL/min flow rate,210 nm] to obtain 3 (5 mg).
Cyclocarioside I (1): Colorless powder (MeOH); [α]D 25: +15.46 (c 0.26,MeOH); NMR data,see Table 1; ESI-MS m/z (%): 627 [M+Na]+; HR-ESI-MS m/z 627.3877 [M+Na]+ (calcd. for C35H56O8Na,627.3867).
| Table 1 NMR spectroscopic data (acetone-d6) for cyclocarioside I (1),cyclocarioside J (2) and cyclocarioside K (3). |
Cyclocarioside J (2): Colorless powder (MeOH); [α]D25: +32.55 (c 0.13,MeOH); NMR data,see Table 1; ESI-MS m/z (%): 645 [M+Na]+; HR-ESI-MS m/z 645.3981 [M+Na]+ (calcd. for C35H58O9Na 645.3973).
Cyclocarioside K (3): Colorless powder (MeOH); [α]D25: +32.55 (c 0.13,MeOH); NMR data,see Table 1; ESI-MS m/z (%): 777 [M+Na]+; HR-ESI-MS m/z 777.4780 [M+Na]+ (calcd. for C41H70O12Na 777.4759). 2.4. Acid hydrolysis
Solution of 1-3 were mixed with 0.5 mol/L HCl and refluxed for 1 h. Each reaction mixture was diluted with water and extracted exhaustively with CHCl3. The aqueous layer was neutralized with sodium bicarbonate and allowed to dry at room temperature. TLC analysis of the residues using CHCl3-MeOH-H2O (6:4:1) showed the presence of arabinose as the only sugar for 1 and 2,and both quinovose and arabinose for 3. 3. Results and discussion
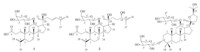
Cyclocarioside I (1) was obtained as colorless powder. The molecular formula of 1 was determined to be C35H56O8 by HR-ESIMS. The 1H NMR spectrum of 1 showed six quaternary methyl singlets of triterpenoid aglycone skeleton at δ 0.97 (s,3H,H3-30), 1.05 (s,3H,H3-18),1.09 (s,3H,H3-19),1.16 (s,3H,H3-21),1.78 (s, 3H,H3-29),and 1.83 (s,3H,H3-27); a pair of coupled each other olefinic protons at δ 5.83 (dt,1H,J = 15.6,8.0 Hz,H-23) and 6.18 (d, 1H,J = 15.6 Hz,H-24),the coupling constant (J = 15.6 Hz) of the olefinic protons showed that the configuration of the double bond is E; two terminal methylene olefinic protons at δ 4.74 (br s,1H,H- 28a),4.84 (br s,1H,H-28b),and 4.86 (br s,2H,H-26); an oxymethine proton at δ 4.16 (dt,1H,J = 10.6,4.4 Hz,H-12). In addition,partially overlapped multiples of methylene and methine protons were showed between δ 0.97 and δ 2.70. These data were used to assign the triterpenoid aglycone skeleton. The presence of an anomeric proton of glycosyl at δ 4.32 (d,1H,J = 6.5 Hz,H-1′),as well as other oxymethine protons and one oxymethylene of glycosyl suggested that 1 was a triterpenoid saponin. The 13C NMR and DEPT spectra of 1 showed 35 carbon signals,30 of the 35 carbons were assigned to the triterpenoid skeleton and 5 to a pentaglucose. It consisted 6 methyls,11 methylenes (two olefinic), 11 methines (two olefinic and five oxygenated),and seven quaternary carbons (one carboxyl,two olefinic,and one oxygenated) (Table 1). According to the molecular formula of 1,there are eight degrees of unsaturation of 1s,including one carboxyl,three double bonds,one ring of glycosyl,three degrees of unsaturation are remained,and it confirmed that 1 possess an abnormal triterpenoid aglycone skeleton with tricyclic parent nucleus,and which was finally established by detailed analyses of 2D NMR spectra of 1 as described below. After NMR signals of protons and protonated carbons (Table 1) were unambiguously assigned by the HSQC experiment,in the HMBC spectrum long range bond heteronuclear correlations (Fig. 2). From H2-28 (4.74 and 4.84, terminal double bond protons) to C-5 (52.3) and C-29 (24.1); from H3-29 (1.78) to C-4 (149.0),C-5 (52.3),and C-28 (113.8); from H2-2 (2.26) to C-1 (38.3) and C-3 (174.4),together with resolvable homonuclear vicinal coupling correlations (1H-1H-COSY) between H2-1 and H2-2; between H-5 and H2-6. These data demonstrated that the circle A of triterpene aglycone of 1 is cleaved to carboxy and terminal double bond at position 3 and 4,and 1 possessed a tricyclic parent nucleus was a novel 3,4-secodammarane triterpene. The monosaccharide obtained by acid hydrolysis was identified by comparision on TLC with authentic sample as arabinose. A further comparison of the NMR data of 1 with those of the related compounds in the literature [15] indicated that 1 were in good agreement with cyclocarin A [(23E)-(12R,20S)-12,20- dihydroxy-3,4-secodammara-4(28),23,25-trien-3-oic acid] except for the data of glycosyl,which was existed in 1,and methoxy group was existed in cyclocarin A. In the HMBC spectrum,the anomeric proton of glycosyl at δ 4.32 (d,1H,J = 6.5 Hz) was correlated with an oxymethine carbon resonances at δ 74.9 (C-12). This result suggested that the glycosyl was located at C-12. The relative configurations at C-12 and C-20 were determined by NOESY correlations between H-12 and H-30,H-21 and H-13,the absolute configurations at C-12 and C-20 were determined to be R and S, respectively,on the basis of comparison to the 13C NMR chemical shift and optical rotation data for analogous 3,4-secodammaranes [6, 16]. Ultimately,the structure of 1 was deduced as (23E)-(12Rs, 20S)-12,20-dihydroxy-3,4-secodammara-4(28),23,25-trien-3-oic acid-12-O-α-L-arabinopyranoside,and named cyclocarioside I.
|
Download:
|
| Fig 2. The key HMBC correlations of compound 1. | |
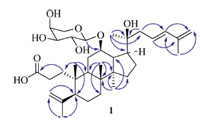
Cyclocarioside J (2) has the molecular formula C35H58O9,as determined by HR-ESI-MS analysis (m/z 645.3981 [M+Na]+). Detailed analysis of the NMR data (Table 1) revealed that the signals of 2 and 1 were nearly identical,except for the signals of the side-chain of the triterpenoid skeleton. In the NMR spectrum of 2, there are one oxymethine proton at δ 3.99 and two olefinic protons at d 4.75 and 4.92 in the side-chain of the triterpenoid skeleton, instead of four olefinic protons at δ 5.83,6.18 and 4.86 (2×) in 1. This suggested that 2 was an addition product of compound 1, add aH2Oon the double bond (C-23) of 1 and formed 24-hydroxy of 2,or 1 was formed by 2 with the dehydration of 24-hydroxy in the biosynthesis pathway. The structure of 2 was confirmed by 2DNMR spectrum,in the HMBC spectrum,the protons of methyl at δ 1.14 (H-21) were correlated with carbon resonances at δ 50.2 (C-17), 74.2 (C-20),and 37.2 (C-22); the protons of methylene at δ 1.46 and 1.57 (H-22) were correlated with carbon resonances at δ 74.2 (C-20) and 75.9 (C-24); the protons of methylene at δ 1.57 and 1.67 (H-23) were correlated with carbon resonances at δ 37.2 (C-22) and 75.9 (C-24); the protons of terminal double bond at d 4.75 and 4.92 (H- 26) were correlated with carbon resonances at δ 18.2 (C-27) and 75.9 (C-24); the proton of methyl at δ 1.83 (H-27) was correlated with carbon resonances at δ 75.9 (C-24),149.4 (C-25),and 110.2 (C- 26) (Fig. 3). The absolute configuration at C-24 was determined to be S,on the basis of comparison to the 13C NMR chemical shift and optical rotation data for analogous 3,4-secodammaranes [6, 16]. Thus the structure of 2 was determined to be (12R,20S, 24S)-20,24-dihydroxy-3,4-secodammara-2(28),25-dien-3-oic acid-12-O-α-L-arabinopyranoside,named cyclocarioside J.

|
Download:
|
| Fig 3. The key HMBC correlations of compound 2. | |
Cyclocarioside K (3) has the molecular formula C41H70O12,as determined by HR-ESI-MS analysis (m/z 777.4780 [M+Na]+). The NMR spectrum of 3 showed that it was a triterpenoid glycoside with a dammarane triterpenoid aglycone skeleton and two sugars (Table 1). Detailed analysis of the NMR data revealed that the signals of 3 and cyclocarioside H [15] were nearly identical,except for the signals of the arabinose. The arabinose and quinovose were identified by acid hydrolysis TLC analysis of 3. The carbon signals of the arabinose,100.2,72.1,73.5,67.9,and 64.8 were identical with the signals of α-L-arabinopyranose of pterocaryoside B [6]. The structure of 3 was confirmed by the HMBC spectrum (Fig. 4). The relative configurations at C-3,C-12,C-20,C-24 were determined by NOESY correlations between H-3 and H-29,H-12 and H-30,H-21 and H-30,H-24 and H-21. The absolute configurations at C-20 and C-24 were determined to be S and R,respectively,on the basis of comparison to the 13C NMR chemical shift for analogous epoxydammaranes [16]. Therefore,the structure of 3 was deduced as (20S,24R)-(3α,12β)-20,24-epoxydammara-25-ol-12-O-β-Dquinovopyranosyl- 3-O-α-L-arabinopyranoside,and 3 was named cyclocarioside K.

|
Download:
|
| Fig 4. The key HMBC correlations of compound 3. | |
Currently,39 secodammarane triterpenoids have been isolated from nature resources,most of which are from genera of family Betulaceae,such as Alnus [17, 18, 19, 20],Dysoxylum [21, 22, 23],Meliaceae, such as Aglaia [24, 25, 26, 27, 28],Cabralea [29],which are distributed in North America,South America,and the north of Australia,and the genus Cyclocarya of Juglandaceae which is distributed in China [5, 6, 15]. The triterpenoid aglycones have anti-RSV [23],anti-HIV- 1[30],cytotoxic [25],antifungal [26] activities. Though no obvious activities were found in the secodammarane triterpenoid saponins, the secodammarane triterpenoid saponins (1 and 2) and epoxydammarane triterpenoid saponin (3) which were isolated from the leaves of C. paliurus (the Chinese common name ‘‘tianyeshu’’, sweet leaf tree) are the main sweet-tasting compounds.
AcknowledgmentWe gratefully acknowledge the financial support of the National Science and Technology Project of China (No. 2012ZX09301002001003).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.11. 033.
| [1] | Editor Committee for Flora of China of the Chinese Academy of Science, Flora of China, vol. 21, Science Publishing House, Beijing, 1979, pp. 18-19. |
| [2] | The Editorial Committee of the Administration Bureau of Traditional Chinese Medicine, Chinese Materia Medica, vol. 2, Shanghai Science and Technology Publishing House, Shanghai, 1999, pp. 370-371. |
| [3] | H. Kurihara, S. Asami, H. Shibata, H. Fukami, T. Tanaka, Hypolipemic effect of Cyclocarya paliurus (Batal) Iljinskaja in lipid-loaded mice, Biol. Pharm. Bull. 26 (2003) 383-385. |
| [4] | R.G. Shu, C.R. Xu, L.N. Li, Studies on the sweet principles from the leaves of Cyclocarya paliurus (Batal.) Iljinsk, Acta Pharm. Sin. 30 (1995) 757-761. |
| [5] | R.G. Shu, C.R. Xu, L.N. Li, Z.L. Yu, Cyclocariosides II and III: two secodammarane triterpenoid saponins from Cyclocarya paliurus, Planta Med. 61 (1995) 551-553. |
| [6] | E.J. Kennelly, L.N. Cai, L.N. Long, et al., Novel highly sweet secodammarane glycosides from Pterocarya puliurus, J. Agric. Food Chem. 43 (1995) 2602-2607. |
| [7] | Z.Y. Jiang, X.M. Zhang, J. Zhou, S.X. Qiu, J.J. Chen, Two new triterpenoid glycosides from Cyclocarya paliurus, J. Asian Nat. Prod. Res. 8 (2006) 93-98. |
| [8] | D.J. Yang, Z.C. Zhong, Z.M. Xie, Studies on the sweet principles from the leaves of Cyclocarya paliurus (Batal.) Iljinskaya, Acta Pharm. Sin. 27 (1992) 841-844. |
| [9] | R.J. Zhong, R.G. Shu, X.L. Ni, C.R. Xu, L.N. Li, Studies on the chemical structure of Cyclocaric acid A, Acta Pharm. Sin. 31 (1996) 398-400. |
| [10] | R.J. Zhong, Y.H. Gao, C.R. Xu, L.N. Li, Pentacyclic triterpenoids from Rounduingfruit Cyclocarya (Cyclocarya paliurus), Chin. Trad. Herbal Drugs 27 (1996) 387-388. |
| [11] | R.G. Shu, Z.R. Song, J.C. Shu, Study on the chemical constituents of the butanol extraction of Cyclocarya paliurus (Batal.) Iljinsk, J. Chin. Med. Mater. 29 (2006) 1304-1307. |
| [12] | R.G. Shu, J.C. Shu, Phenolic constituents of Cyclocarya paliurus, Chin. Trad. Herbal Drugs 38 (2007) 507-508. |
| [13] | J. Li, Y.Y. Lu, F. Li, et al., Study on chemical constituents of Cyclocarya paliurus, J. Chin. Med. Mater. 29 (2006) 441-442. |
| [14] | B.S. Cui, S. Li, Studies on chemical constituents from the leaves of Cyclocarya paliurus, Chin. Trad. Herbal Drugs 43 (2012) 2132-2136. |
| [15] | S. Li, B.S. Cui, Q. Liu, et al., New Triterpenoids from the leaves of Cyclocarya paliurus, Planta Med. 78 (2012) 290-296. |
| [16] | C. Seger, S. Pointinger, H. Greger, O. Hofer, Isoeichlerianic acid from Aglaia silvestris and revision of the stereochemistry of foveolin B, Tetrahedron Lett. 49 (2008) 4313-4315. |
| [17] | T. Aoki, S. Ohta, T. Suga, Six novel secodammarane-tape triterpenes from male flower of Alnus Japonica, Phytochemistry 27 (1988) 2915-2920. |
| [18] | T. Hirata, R. Ideo, T. Suga, Structure of Alnuselide, the first reported naturally occurring C31-secodammarane-type triterpene lactone from Alnus Serrulatoides, Chem. Lett. 6 (1977) 711-714. |
| [19] | A. Tadashi, O. Shinji, A. Satoshi, H. Toshifumi, S. Takayuki, The structures of four novel C31-secodammarane-type triterpenoid saponins from the female flowers of Alnus serrdatoides, J. Chem. Soc. Perkin Trans. 1 (1982) 1399-1403. |
| [20] | T. Suga, S. Ohta, E. Ohta, T. Aoki, A C31-secodammarane-type triterpenic acid, 12- deoxy alnustic acid, from the female flowers of Alnus pendula, Phytochemistry 25 (1986) 1243-1244. |
| [21] | T.R. Govindachari, G. Suresh, G.N. Krishna Kumari, Triterpenoids from Dysoxylum Malabaricum, Phytochemistry 37 (1994) 1127-1129. |
| [22] | L.K. Wah, F. Abas, G.A. Cordell, H. Ito, I.S. Ismail, Steroids from Dysoxylum grande (Meliaceae) leaves, Steroids 78 (2013) 210-219. |
| [23] | S. Yogendra, A. William, Dammarane triterpenoids from Dysoxylum richii, Phytochemistry 31 (1992) 4033-4035. |
| [24] | C.O. Esimone, G. Eck, T.N. Duong, et al., Potential anti-respiratory syncytial virus lead compounds from Aglaia species, Pharmazie 63 (2008) 768-773. |
| [25] | K. Mohamad, T. Sévenet,V. Dumontet, et al., Dammarane triterpenes and pregnane steroids from Aglaia lawii and A. tomentosa, Phytochemistry 51 (1999) 1031-1037. |
| [26] | H. Desi, T. Roekmiati, S. Agus, et al., Cytotoxic triterpenoids from the bark of Aglaia smithii (Meliaceae), Phytochem. Lett. 5 (2012) 496-499. |
| [27] | N. Joycharat, P. Plodpai, K. Panthong, Y. Boonek, P. Supayang, Terpenoid constituents and antifungal activity of Aglaia forbesii seed against phytopathogens, Can. J. Chem. 88 (2010) 937-944. |
| [28] | S. Pointinger, S. Promdang, S. Vajrodaya, et al., Silvaglins and related 2,3-secodammarane derivatives-unusual types of triterpenes from Aglaia silvestris, Phytochemistry 69 (2008) 2696-2703. |
| [29] | M.M. Rao, H. Meshulam, R. Zelnik, D. Lavie, Cabralea eichleriana DC. (Meliaceae) - I Structure and stereochemistry of wood extractives, Tetrahedron 31 (1975) 333- 339. |
| [30] | C.O. Esimone, G. Eck, C.S. Nworu, et al., Dammarenolic acid, a secodammarane triterpenoid from Aglaia sp. shows potent anti-retroviral activity in vitro, Phytomedicine 17 (2010) 540-547. |