b Department of Chemistry and Biochemistry, University of Michigan-Flint, Flint, MI 48502, USA;
c Shenzhen Guanheng New Materials Technology Co., Ltd., Shenzhen 518109, China
Polysiloxane material performs many unique properties over a wide temperature range,such as chemical inertness,good weather resistance,low surface tension and excellent dielectric properties. These special performances allow silicones well suited for applications that experience large temperature swings and long service lifetimes,such as automotive engine sealants,damping material in aircraft and encapsulant for electronic devices [1]. However,polysiloxanes start to degrade around 200℃, especially poly(dimethyl siloxane) undergoes dramatic thermal degradation above 300℃ [1, 2]. Many efforts have been made to improve the high-temperature thermal stability of silicones,such as adding inorganic additive [2],increasing crosslink density [1] and incorporating rigid group (e.g.,phenyl and imide heterocycle) [3, 4]. The inorganic additives are not compatible with silicones, and the processing problems with blending technique still exist [1]. The second method of increasing crosslink density with polymethylmethoxysiloxane as crosslinker is only applicable to silicones vulcanized by free radical polymerization. So the last method has attracted more and more attention in recent years [3, 4]. However,all these rigid groups are derived from the petrochemical route. Maleopimaric acid (MPA) is derived from the natural rosin and can be used to prepare imide compound [5]. Therefore,it could be a good alternative to current petroleumbased rigid monomers [6]. However,there has been no report on the preparation and characterization of imide modified polysiloxane with MPA as the imidization agent. In this work,with MPA as the raw material,MPA based imide heterocycle (MPABI) was grafted onto the side chain of vinyl-containing polysiloxane,and therefore MPA modified silicone (MP-VMS) was obtained and characterized. The curing behavior of MP-VMS was studied by differential scanning calorimetry (DSC). The low-temperature and high-temperature resistance of its curing product were studied by DSC and thermogravimetric analysis (TGA). 2. Experimental
D4(octamethyl cyclotetrasiloxane,purity≥99.5%),D4Vi (1,3,5, 7-tetravinyl-1,3,5,7-tetramethylcyclotetrasiloxane,purity≥97%), MM (hexamethyldisiloxane,purity≥99%),KOH (potassiumhydroxide,purity≥95%) and DBPH (2,5-bis(tert-butylperoxy)-2, 5-dimethylhexane) were purchased from J&K Scientific Ltd. MR (maleated rosin,115) was purchased from Wuzhou Sun Shine Forestry and Chemicals Co.,Ltd. MPA was purified from MR according to literature [6]. HAPMS was obtained from the hydrolyzation of 3-aminopropyl(diethoxy)methylsilane and then dehydrated under vacuum at 100℃for8h.
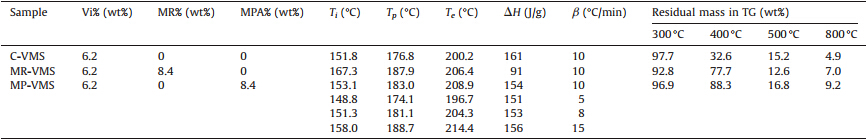
MP-VMS was synthesized by the following steps: Firstly,40 g of D4,5 g of MM,13 g of D4Vi,2.55 g of HAPMS and 0.1 wt% of KOH were mixed and dehydrated,and then heated to 100℃; after 4 h, 5.55 g of MPA was added and the mixture was slowly heated to 150℃ in about 4 h; finally,the system was pumped into vacuum to remove the volatile. The preparation scheme of MP-VMS was shown in Fig. 1. Preparation scheme of MP-VMS,the NMR results are listed as following: 1H NMR (300 MHz,CDCl3) [5]:δ5.6-6.2 (Si- CH=CH2),5.4 (CH=C in MPABI),3.3 (CHC55O in MPABI),3.0 (CH2- N),0.3-2.8 (Si-CH2-CH2and the other protons in MPABI),0-0.3 (Si-CH3); 13C NMR (75 MHz,CDCl3) [5, 7]:δ184 (COOH),176-179 (O55C-N-C55O in MPABI),145 (CH=C in MPABI),133,138 (Si- CH=CH2),124 (CH=C in MPABI),15-55 (Si-CH2CH2CH2-N and the other carbons in MPABI),8.5 (SiCH2),0.7 (SiCH3),-1.5 (SiCH3).
|
Download:
|
| Fig. 1. Preparation scheme of MP-VMS | |
As a contrast,MR modified vinyl-containing silicone (MR-VMS) was prepared strictly according to the aforementioned process, except that MPA was replaced by MR. Common vinyl-containing silicone (C-VMS) was also prepared according to the aforementioned process,except that HAPMS and MPA were absent and the loading of D4was adjusted to 48.1 g. The NMR results of C-VMS are listed as following: 1H NMR (300 MHz,CDCl3):δ 5.6-6.2 (Si- CH=CH2),0-0.3 (Si-CH3); 13C NMR (75 MHz,CDCl3):δ133-138 (Si-CH=CH2),0.7 (SiCH3).
NMR analyses were conducted with a Bruker DRX-500 nuclear magnetic resonance spectrometer with tetramethylsilane as the internal standard. DSC was performed on a Perkin-Elmer Diamond instrument in flowing nitrogen gas. TGA was performed on a TG209F1 thermogravimetric analyser at a heating rate of 10℃/min in a nitrogen flow. The mathematical model for curing reaction of MP-VMS was represented in the form of Eq. (1) [8], where the reaction constantk(T) was expressed by the Arrhenius equation (see Eq. (2)) [8]. Activation energy (Ea,k) and preexponential factor (A) were determined by Kissinger method [9], which was based on the Kissinger equation (see Eq. (3)) [9]. Activation energy (Ea,O) were determined by Ozawa plot [9], which was based on the Ozawa equation (see Eq. (4)) [9]. The activation energy (Ea) of MP-VMS was ultimately represented by the average value of Ea,k and Ea,o(i.e.,Ea=(Ea,k+Ea,o)/2). Reaction order (n) was determined by the Crane equation (see Eq. (5)) [9].
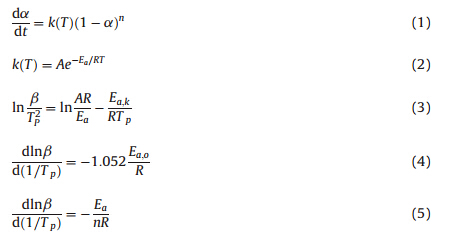
With the great difference in molecular polarity,MPA or MR could not dissolve in polysiloxane to form a homogeneous solution. However,the obtained MP-VMS was homogeneous,which strongly demonstrated the formation of chemical bonding between MPA (or MR) and silicone polymer. In order to study the chemical grafting reaction,C-VMS and MP-VMS were characterized by NMR. The NMR spectra of MP-VMS were more complex than C-VMS. And the characteristic peaks of MPABI could be observed in the 1H NMR and 13C NMR spectra. These signals, especially the peaks of CH=C,O=C-N-C=O and CH2-N,strongly proved that MPABI had been successfully grafted onto the side chain of polysiloxane polymer by the imidization reaction.
Initiated by 1.4 wt% of DBPH,the DSC results of MP-VMS,MRVMS and C-VMS were presented in Table 1. DSC results of MP-VMS, MR-VMS and C-VMS,and the TG results of their curing products, where Vi% (MR% or MPA%) indicated the weight content of vinyl group (MR or MPA) in the formula of VMS. With the same heating rate (β),the initial temperature (Ti ),Tp and end temperature (Te )of exothermic peaks of both MR-VMS and MP-VMS were higher than those of C-VMS,while their exothermic heat (DH) were less than that of C-VMS. It seemed that incorporation of MPA or MR in polysiloxane could inhibit the vulcanization of vinyl containing silicone resin,which should be ascribed to the trace amount of rosin acid remained in MR-VMS or MP-VMS. Since MR was produced from rosin acid [6],MR-VMS contained trace amounts of rosin acid,which acted as a radical scavenger [10]. In this study, MPA was purified from MR,and therefore MP-VMS contained less amount of rosin acid than MR-VMS. As a result,DHof MP-VMS was more than that of MR-VMS. Based on the DSC data of MP-VMS (β= 5,8,10,15℃/min),the kinetic parameters in the curing reaction of MP-VMS,including Ea,Ea,k,Ea,o,A and n,were respectively calculated as 18.6 kJ/mol,17.6 kJ/mol,19.6 kJ/mol, 71,108 and 0.902.
| Table 1 DSC results of MP-VMS,MR-VMS and C-VMS,and the TG results of their curing products |
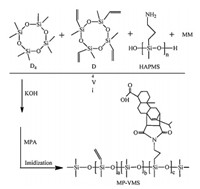
The thermal stability of the curing products from MP-VMS,MRVMS and C-VMS were measured by TGA in the temperature range from 25℃ to 800℃ (Fig. 2,TG and DTG curves of the curing products from C-VMS (a),MR-VMS (b) and MP-VMS (c)),and the residual masses at different temperatures were presented in Table 1. DSC results of MP-VMS,MR-VMS and C-VMS,and the TG results of their curing products. Before 300℃,all specimens performed a similar thermal stability,and the maximum difference value among them did not exceed 5%. Their obvious weight losses began around 350℃ and were complete by 600℃. At 400℃,the residual mass from MP-VMS was 55.7% higher than that from CVMS,while MR-VMS was 45.1% higher than C-VMS. The values of Tmax(the temperature corresponding to the weight-loss ratemaximum in the DTG curve) were respectively 368.2℃ (C-VMS), 425.2℃ and 439.9℃ (MR-VMS),and 438.9℃ (MP-VMS). It could be observed that the curing product of MP-VMS exhibited the most excellent thermal stability,which should be ascribed to the incorporation of imide heterocycle [4]. Since the purity of MPA was higher than MR,the content of MPABI in MP-VMS was higher than that in MR-VMS. As a result,the DTG curve from MR-VMS exhibited a bimodal wave,and the thermal stability of MP-VMS was higher than MR-VMS.

|
Download:
|
| Fig. 2. TG and DTG curves of the curing products from C-VMS (a),MR-VMS (b) and MP-VMS (c) | |
The low-temperature resistances of their curing products were also investigated by DSC in the temperature range from -70℃to 0℃. All of the DSC curves showed a similar change trend,and neither glass transition nor the other phase changes had been observed. This result indicated that the curing products were stable in this wide temperature range. So their glass transition temperatures (Tg) should be lower than -70℃,below which elastomer would lose its elasticity and become a plastic. In sum, incorporation of MPABI had no effect on the low-temperature resistance of silicone resin in the temperature range from -70℃ to 0℃. 4. Conclusion
With MPA as a raw material,imide heterocycle (MPABI) could be incorporated into polysiloxane resin,and MP-VMS was obtained and characterized by 1H NMR and 13C NMR. The curing kinetic parameters of MP-VMS including Ea,Aandn,were respectively 18.6 kJ/mol,71,108 and 0.902. The TGA and DSC results showed that incorporation of MPABI could significantly improve the thermal stability of silicone resin while had no effect on the lowtemperature resistance,and the Tmax of the curing product from MP-VMS increased by 70.7℃.
| [1] | Y. Han, J.Y. Zhang, L. Shi, et al., Improvement of thermal resistance of polydimethylsiloxanes with polymethylmethoxysiloxane as crosslinker, Polym. Degrad. Stab. 93 (2008) 242-251. |
| [2] | Y.Y. Yao, G.Q. Lu, D.S. Boroyevich, et al., Effect of Al2O3 fibers on the hightemperature stability of silicone elastomer, Polymer 55 (2014) 4232-4240. |
| [3] | Y.H. Shi, X.X. Gao, D. Zhang, et al., Synthesis and thermal properties of modified room temperature vulcanized (RTV) silicone rubber using polyhedral oligomeric silsesquioxane (POSS) as a cross linking agent, RSC Adv. 4 (2014) 41453-41460. |
| [4] | C. Hamciuc, E. Hamciuc, D. Serbezeanu, et al., Phosphorus-containing poly(esterimide)- polydimethylsiloxane copolymers, Polym. Int. 60 (2011) 312-321. |
| [5] | J. Wang, Y.P. Chen, K. Yao, et al., Robust antimicrobial compounds and polymers derived from natural resin acids, Chem. Commun. 48 (2012) 916-918. |
| [6] | X. Xu, S.B. Shang, Z.Q. Song, et al., Preparation and characterization of rosin-based waterborne polyurethane from maleopimaric acid polyester polyol, Bioresources 6 (2011) 2460-2470. |
| [7] | Y. Ma, Q.Y. Tang, J. Zhu, et al., Fluorescent and thermal properties of siloxane- polyurethanes based on 1,8-naphthalimide, Chin. Chem. Lett. 25 (2014) 680-686. |
| [8] | R.N. Jana, H.P. Bhunia, G.B. Nando, An investigation into the mechanical properties and curing kinetics of blends of low-density polyethylene and polydimethyl siloxane rubber, Thermochim. Acta 302 (1997) 1-9. |
| [9] | X.L. Zhao, Study of curing behaviors of vinyl ester resin, Master’s thesis of Inner Mongolia University of Technology, 2010, pp. 30-33. |
| [10] | P.X. Chen, Preparation and properties of rosin derivative/acrylate composite emulsion and its pressure sensitive adhesive with high temperature resistance, Doctor’s thesis of South China University of Technology, 2012, p. 22. |