b Department of Chemistry, Dezful Branch, Islamic Azad University, Dezful, Iran
Azlactones,or 2,4-substituted oxazolin-5-ones,are an important classes of five membered heterocyclic compounds containing nitrogen and oxygen as hetero atoms,which have attracted much interest due to their wide range of biological and pharmaceutical properties [1, 2, 3]. They are appropriate building blocks and valuable intermediates for preparation of diverse biologically active molecules,including amino acids [4, 5],peptides [6],heterocyclic compounds [7] as well as biosensors or coupling and photosensitive devices for proteins [8]. Furthermore,these compounds are especially active as anticancer [9],antitumor [10],antimicrobial [11],anti-inflammatory [12],anti-hypertensive [13],and inhibitor of central nervous system. The most well-known route for the azlactone synthesis is the Erlenmeyer method. The Erlenmeyer reaction was first described in 1893 by Friedrich Gustav Carl Emil Erlenmeyer [14] who reported the condensation of benzaldehyde withN-acetylglycine in the presence of sodium acetate as a basic catalyst in acetic anhydride as a dehydrating agent. After the Erlenmeyer report,several other dehydrating agent and catalysts such as perchloric acid [15],polyphosphoric acid [16],Al2O3-H3BO3 [17],supported KF [18],Bi(OAc)3 [19],Bi(OTf)3 [20],Ca(OAc)2 [2], anhydrous zinc chloride [21],Yb(OTf)3 [22],Al2O3[23],POCl3 [24], [C6(MIm)2]2W10O32·2H2O[25],Nano silica supported tungstophosphoric acid [26] and TsCl-DMF [27] have been employed for condensation of carbonyl compounds and hippuric acid. However, some of these procedures have some drawbacks,such as unsatisfactory yields,use of water-sensitive catalysts and nonreusable catalysts,which generate toxic waste streams and difficult product separation. Therefore,the development of a reusable,ecofriendly and more convenient catalyst for the synthesis of azlactones without using any hazardous solvents is still demand.
In recent years,the green chemistry has been become an interesting research field for organic chemists. Among the different strategies for achieving this goal,the use of heterogeneous catalysts and solvent-free methods which lead to elimination of solvent and waste,has received special attentions. Heterogeneous catalysts compared to their homogeneous counterparts have some inherent advantages such as milder experimental conditions,an easier set-up and work-up,easy removal,recycling and reuse of catalysts. Recently,most attempts have focused on the anchoring of soluble active species on an insoluble matrix as heterogeneous catalysts for many reactions,such as epoxidation and alkylation [28],hydrogenation [29],Michael reaction [30],aldol reaction [31], sulphide oxidation [32],and esterification [33]. Among the best studied and most frequently employed supported reagents,SiO2 [34, 35, 36] has been a focus of extensive research due to its chemical stability,non-toxicity,cheap,environmentally friendly and abundant. Furthermore,SiO2 nanoparticles showed their potential in many fields,such as catalyst or catalyst support,due to the higher surface area and lower coordinating sites,which lead to the higher catalytic activity [26, 37, 38, 39, 40, 41]. As a part of our ongoing research on the development of useful synthetic protocols [42, 43, 44],herein,we wish to explore a straight forward convergent microwave-assisted synthesis of azlactone derivativesviathe one-pot condensation of carbonyl compounds and hippuric acid under solvent-free conditions using nano-sphere silica supported 2-aminopyridine (nanoSiO2 -AP) as an proficient,harmless to the environment,mild and solid base catalyst with good stability (Scheme 1).
|
Download:
|
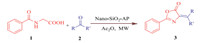
| Scheme 1.The Erlenmeyer reaction pathway catalyzed by 2-aminopyridinefunctionalized sphere SiO2 nanoparticles. | |
All chemicals and solvents were obtained from commercial sources (Aldrich-Sigma and Merck chemical companies). All known organic products were identified by comparison of their physical and spectral data with those of authentic samples. The FTIR spectra of the sample were recorded on a Unicom Galaxy Series FT-IR 5000 spectrophotometer using pressed KBr discs. The NMR spectra were recorded on a Brucker Avance spectrometer using DMSO-δ6or CDCl3with TMS as an internal standard. The X-ray diffraction (XRD) was performed on Philips X-Pert (Cu Ka radiation,λ=0.15405 nm) in the range of 2θ=10°-70° using 0.048as the step length. Thermal Gravimetric Analysis (TGA) data for nano-SiO2 -AP particles was taken on a Mettler TA4000 System under N2atmosphere at rate of 10℃/min. Transmission electron microscope,TEM (Philips CM-10) was also used to obtain TEM images. 2.1. Synthesis of nano-sphere SiO2
Nano-sphere SiO2 was prepared according to the reported procedure with slight modifications [45]. Briefly,a mixture of ethanol (100 mL),deionized water (20 mL) and 1.0 g of polyethylene glycol (MW 6000) was dispersed by ultra-sonication. Then, aqueous ammonia (25%,2.5 mL) and tetraethyl orthosilicate (TEOS, 2.0 mL) was added to the reaction mixture. The resulting dispersion was stirred magnetically for 24 h under reflux conditions. A white precipitation was separated from the reaction medium by centrifugation at 4000 rpm for 10 min,washed with hot ethanol,then with deionized water followed by the overnight vacuum-drying at 70℃. 2.2. Synthesis of 3-chloropropyl-functionalized nano-sphere SiO2 (nano-SiO2 -Cl)
Chloropropyl-modified nano-sphere SiO2 was prepared according to the Zenget al.procedure [46]. Nano-sphere SiO2 (1.0 g) was added to the solution of 2.0 mL of 3-chloropropyl-trimethoxysilane (CPTS) in 100 mL of dried toluene and dispersed by ultrasonication. Then,the dispersed mixture was stirred for 24 h at 60℃. Chloropropyl-functionalized solid (nano-SiO2 -Cl) was separated by centrifugation,washed with toluene,and dried under vacuum at 70℃. 2.3. Synthesis of 2-aminopyridine-functionalized nano-sphere SiO2 (nano-sphere SiO2 -AP)
2-Aminopyridine functionalized SiO2 nanoparticles were prepared by means of a procedure reported elsewhere with little modification [47]. A 1.0 g sample of nano-SiO2 -Cl was dispersed in 20 mL dry toluene by ultrasonic bath for 20 min. To this suspension,1.2 g of 2-aminopyridine and 0.50 mL of triethylamine was added. The resulting mixture was refluxed at 110℃ in an oil bath for 24 h. Then,the as-prepared functionalized SiO2 nanoparticles were separated by centrifugation and washed with Chloroform,then with EtOH,and finally with distilled water. The solid sample was then dried under vacuum at 100℃ for 24 h. 2.4. General procedure for the microwave-assisted synthesis of azlactone derivatives catalyzed by nano-sphere SiO2 -AP
To a mixture of appropriate aldehyde or ketone (1.0 mmol), hippuric acid (0.22 g,1.2 mmol),Ac2O (0.31 mL,3 mmol) and catalyst (0.05 g,4.2 mol% AP) was added. Then,the reaction mixture was irradiated using the microwave oven at a power output of 300 W for the appropriate time according to Table 2. After completion of the reaction as indicated by TLC,the reaction mixture was cooled to room temperature. Cold ethanol (5 mL) was added and the mixture was stirred for 10 min until a yellow solid precipitated. An aqueous solution of NaHCO3 (10 mL,20%) was added,the solid products and the catalyst were filtered. The solid materials were dissolved in hot ethanol and filtered to remove the catalyst. The solvent was evaporated and the crude products were recrystallized from ethanol or mixture of EtOH-H2o.
Spectral data for new compounds are listed below:
(Z)-4-(4-Formylbenzylidene)-2-phenyloxazol-5(4H)-one (3o): IR (KBr,cm-1 ): vmax3072,1797,1751,1701,1653,1553,1491, 1451,1327,1298,1211,1161,982,868,852,696; 1H NMR (300 MHz,CDCl3):δ10.01 (s,1H,CHO),8.29 (d,2H,J= 8.4 Hz),8.15 (d,2H,J= 6.9 Hz),7.91 (d,2H,J= 8.4 Hz),7.59 (t,1H,J= 7.3 Hz), 7.50 (t,2H,J= 7.4 Hz),7.18 (s,1H,-CH=); 13C NMR (75 MHz, CDCl3):δ 191.6 (CHO),165.1(C=O),163.7 (C55N),139.0,137.1, 135.6,134.0,132.7,129.9,129.2,129.1,128.7,125.2; Anal. Calcd. for C17H11NO3: C,73.64; H,4.00; N,5.05. Found: C,73.71; H,4.06; N,4.98.
4-(4-(tert-Butyl)cyclohexylidene)-2-phenyloxazol-5(4H)-one (3r): IR (KBr,cm-1 ): vmax3051,2971,2945,2866,1786,1755, 1660,1572,1489,1451,1366,1321,1192,1152,978,883,781, 702; 1H NMR (300 MHz,CDCl3):δ8.06 (d,2H,J= 7.5 Hz),7.58-7.47 (m,3H),4.03 and 3.59 (AB system,2H,J= 13.3 Hz,CH2),2.21-2.08 (m,4H,CH2and CH),1.41-1.25 (m,3H,CH2),0.91 (s,9H,CH3); 13C NMR (75 MHz,CDCl3):δ165.6 (C=O),161.2 (C55N),159.2,132.3, 128.8,128.7,127.6,126.2,47.5 (CH2),32.5(C),32.0,28.9,27.6 (CH3); Anal. Calcd. for C19H23NO2: C,76.73; H,7.80; N,4.71. Found: C,76.78; H,7.85; N,4.68.
N-Acetyl-N-(1,3-dioxo-1,3-dihydrobenzo[c]oxepin-4-yl)benzamide (3s): IR (KBr,cm-1 ):vmax3080,1811,1773,1682,1651, 1605,1452,1410,1310,1198,1149,1090,1051,1028,995,870, 764,742,687; 1H NMR (300 MHz,CDCl3):δ8.37 (d,1H,J= 7.9 Hz), 7.79 (d,2H,J= 6.5 Hz),7.70-7.66 (m,3H),7.47 (d,4H,J= 5.3 Hz), 2.28 (s,3H,CH3); 1H NMR (300 MHz,DMSO-δ6):δ8.20 (d,1H, J= 8.0 Hz),8.07 (d,1H,J= 7.9 Hz),7.93 (t,1H,J= 8.0 Hz),7.87 (s,1H), 7.78-7.70 (m,3H),7.52-7.48 (m,3H),2.27 (s,3H,CH3); 13C NMR (75 MHz,CDCl3):δ168.2 (C=O),160.2 (C=O),160.1 (C=O),157.7 (O-C=O),135.8,133.7,133.4,130.8,130.1,129.0,128.6,128.5, 128.3,126.1,125.9,108.0,21.7 (CH3); Anal. Calcd. for C19H13NO5:C, 68.06; H,3.91; N,4.18. Found: C,68.11; H,3.88; N,4.23. 3. Results and discussion 3.1. Preparation and characterization of the catalyst
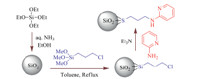
The catalyst was prepared by covalent attachment of the 2-aminipyridine onto the surface of the nano-silica (Scheme 2). At first,nano-sphere silica was prepared from hydrolysis of tetraethylorthosilicate (TEOS) in ethanol with ammonia as a catalyst according to the reported procedure [45]. Then,(3-chloropropyl) triethoxysilane was treated with nano-sphere silica,where the binding between the groups occurs through covalent bonds giving 3-chloropropyl nano-silica. The nucleophilic substitution of the chloride with 2-aminipyridine gave 3-(2-aminipyridine) propyl nano-sphere silica (nano-sphere SiO2 -AP).
|
Download:
|
| Scheme 2.Preparation steps for fabricating of 2-aminopyridyne-functionalized sphere SiO2 nanoparticles. | |
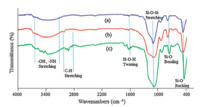
Fig. 1 shows the FT-IR spectrum of nano-sphere SiO2 ,nanosphere SiO2 -Cl,and nano-sphere SiO2 -AP particles in the wavenumber range of 4000-400 cm-1 . There are broad absorption bands at 1100,950,800 and 470 cm-1 attributed to the asymmetric stretching,symmetric stretching,in plane bending and rocking mode of the Si-O-Si group,respectively. The results indicate the presence of SiO2 . The broad peaks in the range of 3200-3500 cm-1 and the very weak peak at 1620 cm-1 are due to the stretching vibration mode of O-H (Si-OH) and twisting vibration mode of H-O-H adsorbed in silica,respectively. The absorption bands in the region of 2850-2990 cm-1 are corresponded to the C-H stretching vibrations (curves b and c). In the FT-IR spectra of nano-sphere SiO2 -AP (Fig. 1; c),in addition to the above mentioned vibrations,the band at 1650 cm-1 (C55N) is a good indication for the presence of 2-aminopyridine fragment on the nano-silica.
|
Download:
|
| Fig. 1. The comparative FT-IR spectra for (a) SiO2 ,(b) SiO2 -Cl and (c) SiO2 -AP nanoparticles. | |
Fig. 2 shows the XRD pattern of nano-sphere SiO2 -AP catalyst prepared by hydrolysis of TEOS. It is clear that the structure of sample was different from that of SiO2 crystalloid. The as-prepared SiO2 particles,which only showed a broad diffraction band at 2u 238are amorphous with no crystallized phases.

|
Download:
|
| Fig. 2. The XRD pattern of 2-aminopyridyne-functionalized sphere SiO2 nanoparticles with a characteristic amorphous peak | |
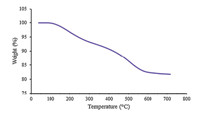
TGA analyses of the nanoparticles were also used to determine the percentage of organic functional groups chemisorbed onto the surface of nano-sphere SiO2 -AP catalyst (Fig. 3). It shows a twostep weight loss over the range of 30-700℃. The first stage including a low amount of weight loss at T<250℃ is due to the removal of physically adsorbed solvent and surface hydroxyl groups. The second stage (250-700℃) is due to the decomposition of 2-aminopyridine binding onto silica spheres chemically. The amount of organic moiety was found to be about 11.5 wt% against total solid catalyst. This result showed that the total amount of organic moieties on nano-SiO2 was about 0.84 mmol/g.
|
Download:
|
| Fig. 3. The TGA curve of 2-aminopyridyne-functionalized sphere SiO2 nanoparticles at heating rate of 108C/min under N2. | |
Fig. 4 shows TEM images of the amorphous nano-sphere SiO2 - AP particles at different optical magnifications. The results confirm the formation of the catalyst,with spherical morphology and a size range of 80-140 nm.

|
Download:
|
| Fig. 4. TEM images of 2-aminopyridyne-functionalized sphere SiO2 nanoparticles at different optical magnifications (Philips CM-10 at 150 kV). | |
After characterizations of nano-sphere SiO2 -AP catalyst, experiments were performed to optimize the reaction conditions for synthesis of azlactones (Scheme 1). Initially,as a model,the condensation reaction of benzaldehyde,hippuric acid and acetic anhydride was examined in the presence of nano-sphere SiO2 -AP in different solvents and also under solvent-free condition using classical thermal heating and microwave irradiation (Table 1). Recently,there has been a growing interest in organic reaction without solvent and under nonconventional conditions such as ultrasound or microwave irradiation. Rate enhancements and large decrease in reaction times,the cleaner and better yields of reactions are some advantages of microwave irradiation [48]. Also, microwave heating makes the process more efficient in the absence of any organic solvents.
| Table 1 Optimization of the reaction conditions for synthesis of 3a.a |
As shown in Table 1,it was found that microwave-assisted solvent-free condition is more efficient (Table 1,entry 6) over the organic solvents and conventional heating under solvent-free condition with respect to reaction time and yield of the desired azlactone. When the reaction was carried out in the presence of different amounts of catalysts,the highest yield was obtained with 0.05 g of catalyst under microwave-assisted solvent-free condition after 3 min. Increasing the amount of catalyst to 0.08 g did not affect the product yield (Table 1,entry 7). Moreover,the catalyst is essential and in the absence of the catalyst,only 18% of the corresponding azlactone was produced even after prolong reaction times (Table 1,entry 4). The efficiency of nano-sphere SiO2 , unbound aminopyridine/nano-SiO2 and free 2-aminopyridine to serve as a catalyst toward the model reaction has been compared and the result are depicted in Table 1. It was observed that the nano-sphere SiO2 -AP was more efficient than the nano-sphere SiO2 ,unbound aminopyridine/nano-SiO2 and free 2-aminopyridine catalysts (Table 1,entry 11).
In order to study the generality of this procedure,a wide variety of aromatic aldehydes bearing electron-withdrawing and electrondonating groups were reacted with hippuric acid and Ac2O in the presence of nano-sphere SiO2 -AP under microwave-assisted solvent-free conditions. The results (Table 2) showed that the corresponding azlactones were obtained in 83%-95% isolated yield in 2-12 min. As can be seen from Table 2,the procedure was highly effective and the nature of substituents on the aromatic ring did not show obvious effects in terms of yields under the reaction conditions. It is worth mentioning that heterocyclic aldehydes such as pyridine-2-carbaldehyde,5-metylthiophene-2-carbaldehyde and thiophene-2-carbaldehyde took part smoothly in the reaction to give the desired products in 89%-92% yields (Table 2, entries 11-13). Moreover,dialdehyde such as terephthaldialdehyde was reacted with 2 mmol of hippuric acid in the presence of a catalytic amount of nano-sphere SiO2 -AP under conventional conditions to afford the corresponding symmetrical bis-azlactone in high yield (Table 1,entry 14). It is noteworthy that,when 1 mmol of hippuric acid was used in the reaction with terephthaldialdehyde in the presence of this catalyst,the desired product which contains one azlactone species was produced in 94% yield (Table 1,entry 15).
| Table 2 Synthesis of azlactone derivatives catalyzed by (Nano-sphere SiO2 -AP) under microwave irradiation |
In order to further expand the scope of this catalytic system, cyclic ketones such as cyclopentanone,cyclohexanone and 4-(tBu)-cyclohexanone were used and successfully converted to their corresponding azlactone derivatives with moderate yields (Table 2, entries 16-18). Aliphatic aldehydes such as acetaldehyde or propionaldehyde and ketones such as ethyl methyl ketone or acetophenone were also examined under the same conditions,but the corresponding products were isolated only in trace amounts.
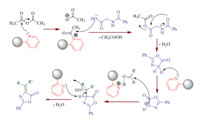
A plausible mechanism for the formation of azlactones catalyzed by nano-sphere SiO2 -AP is shown in Scheme 3. We propose that the reaction proceedsviathe initial activation of the carboxyl group of hippuric acid by [nano-sphere SiO2 -AP: Ac2O] complex,followed by cyclization at the oxygen center to form 2-phenyl-5-oxazolone intermediate that supported by good evidence in Erlenmeyer synthesis [23, 54, 55]. This is then apparently deprotonated by nano-sphere SiO2 -AP to form oxazolone anion which adds to the activated carbonyl compounds (by protonated catalyst) produces the corresponding product. It seems that microwave irradiation affects the stability of the intermediates and therefore,accelerates the reaction accordingly.
|
Download:
|
| Scheme 3.The possible mechanism for catalytic activity of nano-sphere SiO2 -AP toward the synthesis of azlactone derivatives. | |
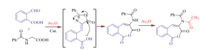
It is noteworthy that,the condensation of 2-formylbenzoic acid and hippuric acid under optimized reaction conditions was produced benzo[c]oxepine-1,3-dione derivative as an unexpected product. Proposed route for the formation of this product illustrated in Scheme 4. First,condensation between the 2-formylbenzoic and the hippuric acid in the presence of the catalyst affords the corresponding azlactone as an intermediate. Then,the observed product can be obtained from the re-cyclizationviaopening of the azalactone ring by carboxyl group (similar to 4-alkylaminocoumarin-3-carbaldehydes [56]) and formation of the fused oxepine ring,followed by acetylation of NH group. The structure of this compound was confirmed with FT-IR,H NMR and C NMR techniques.
|
Download:
|
To compare the applicability and the efficiency of our catalyst with the reported inorganic or organic catalysts for the synthesis of azlactones,we have tabulated the results of these catalysts to perform the condensation reaction of benzaldehyde and hippuric acid under optimized conditions in Table 3. It showed that the nano-sphere SiO2 -AP was a fairly good catalyst for this reaction in terms of short reaction time and simplified conditions. Also,it is seen in addition to having the general advantages attributed to the heterogeneous catalysts,nano-sphere SiO2 -AP is an equally or more efficient catalyst for this condensation reaction.
| Table 3 Comparison of nano-sphere SiO2 -AP with other systems reported in the literature for the synthesis of (Z)-4-benzylidene-2-phenyloxazol-5(4H)-onea |
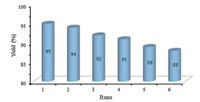
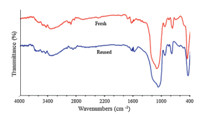
The recovery and reusability of the catalyst are very important for commercial and industrial applications as well as green process considerations. Thus,the recyclability of nano-sphere SiO2 -AP was investigated by using 3-nitrobenzaldehdye,hippuric acid,and Ac2O as model substrates. The catalyst was recovered easily by simple filtration,washed exhaustively with hot ethanol and dried under vacuum and reused in a subsequent reaction. Nearly quantitative catalyst (up to 98%) could be recovered from each run. As seen in Fig. 5,the catalyst showed no substantial reduction in the activity even after six runs. The recovered catalyst after six runs had no obvious change in structure of the catalyst and the characteristic bands,referring to the FT-IR spectra in comparison with the fresh one (Fig. 6). Furthermore,the nitrogen content of fresh SiO2 -AP and recovered catalyst was determined by elemental analysis,showing an amount of organic moiety of about 0.78 and 0.77 mmol/g of nano-silica respectively.
|
Download:
|
| Fig. 5. .Recyclability of nano-sphere SiO2 -AP in the reaction of 3-nitrobenzaldehdye (1 mmol),hippuric acid (1.2 mmol),Ac2O (3 mmol) and catalyst (0.05 g) under microwave irradiation | |
|
Download:
|
| Fig. 6. .The FT-IR spectrum for the comparison of the fresh catalyst and the six-times reused catalyst. | |
In conclusion,the nano-sphere silica supported 2-aminopyridine (nano-sphere SiO2 -AP) was prepared by a multiple synthetic procedure which is confirmed with FT-IR,XRD,TGA,and TEM. This catalyst was successfully applied for the microwave-assisted synthesis of azlactone derivativesviathe condensation of hippuric acid with a wide variety of aromatic,heteroaromatic and cyclic ketones under solvent-free conditions. High catalytic activity, generality,high yields,very short reaction times and the simple experimental procedure combined with the ease of work-up of the product in the absence of any toxic organic solvents make this method quite convenient and environmentally benign for synthesis of azlactones. Furthermore,the catalyst is stable under the reaction conditions and can be recycled and reused several times without a significant loss in activity.
AcknowledgmentWe gratefully acknowledge the financial support from the Research Council of Arak University.
| [1] | K. Takenaka, T. Tsuji, Synthesis of [1,3,4]thiadiazolo[3,2-a]pyrimidines in the presence of formic acid, J. Heterocyclic. Chem. 33 (1996) 1367-1370. |
| [2] | S. Paul, P. Nanda, R. Gupta, A. Loupy, Calcium acetate catalyzed synthesis of 4- arylidene-2-phenyl-5(4H)-oxazolones under solvent-free conditions, Tetrahedron Lett. 45 (2004) 425-427. |
| [3] | K. Mohammed Khan, M.R. Mughal, M.T. Hassan Khan, et al., Oxazolones: new tyrosinase inhibitors; synthesis and their structure-activity relationships, Bioorg. Med. Chem. 14 (2006) 6027-6033. |
| [4] | J.T. Konkel, J. Fan, B. Jayachandran, K.L. Kirk, Syntheses of 6-fluorometa-tyrosine and of its metabolites, J. Flouorine Chem. 115 (2002) 27-32. |
| [5] | S. Chandrasekhar, P. Karri, Aromaticity in azlactone anions and its significance for the Erlenmeyer synthesis, Tetrahedron Lett. 47 (2006) 5763-5766. |
| [6] | F. Cavalier, J. Verducci, New synthesis of the cyclic tetrapeptide tentoxin employing an azlactone as key intermediate, Tetrahedron Lett. 36 (1995) 4425-4428. |
| [7] | A. Avenoza, J.H. Busto, C. Cativiela, J.M. Peregrina, Reactivity of (Z)-4-arylidene- 5(4H)-oxazolones: [4 + 2] cycloaddition versus [4 + 3] cycloaddition/nucleophilic trapping, Tetrahedron Lett. 43 (2002) 4167-4170. |
| [8] | G.T. Hermanson, G.R. Mattson, R.I. Krohn, Preparation and use of immunoglobulin- binding affinity supports on Emphaze beads, J. Chromatogr. A 691 (1995) 113- 122. |
| [9] | E. Etschenberg, H. Jacobi, W. Opitz, Ger. Pat., 904512 (1980). |
| [10] | C. Sanchez, C. Mendez, J.A. Salas, Indolocarbazole natural products: occurrence, biosynthesis, and biological activity, Nat. Prod. Rep. 23 (2006) 1007-1045. |
| [11] | S.A. Siddiqui, S.R. Bhusare, D.V. Jarikote, R.P. Pawar, Y.B. Vibhute, New novel synthesis and antibacterial activity of 1-(substituted phenyl)-2-phenyl-4-(3'- halo, 4'-hydroxy 5'-methoxy benzylidene)-imidazole-5-ones, Bull. Korean Chem. Soc. 22 (2001) 1033-1036. |
| [12] | U. Salgın-Goksen, N. Gö khan-Kelekci, O. Goktas, et al., 1-Acylthiosemicarbazides, 1,2,4-triazole-5(4H)-thiones, 1,3,4-thiadiazoles and hydrazones containing 5- methyl-2-benzoxazolinones: synthesis, analgesic-anti-inflammatory and antimicrobial activities, Bioorg. Med. Chem. 15 (2007) 5738-5751. |
| [13] | K. Urano, Y. Tornioka, K. Okubo, K. Yarnazaki, A. Nagamatsu, Preparation of 4-[(alkylamino)alkylidene]-2-phenyl-2-oxazolin-5-ones, Jpn. Kokai Tokkyo Koho JP 01 29369 189 29, 3691, (1989). |
| [14] | E. Erlenmeyer, Ueber die condensation der hippursäure mit phtalsäureanhydrid und mit benzaldehyd, Annalen 275 (1893) 1-8. |
| [15] | G.V. Boyd, P.H. Wright, Cyclisation of α-acylamino-acids in the presence of perchloric acid to give 5-oxo-△2-oxazolinium perchlorates, J. Chem. Soc., Perkin Trans. 1 (1972) 909-913. |
| [16] | Y.S. Rao, Reactions in polyphosphoric acid. I. New stereospecific synthesis of the E isomers of 2-phenyl-4-arylmethylene-2-oxazolin-5-ones, J. Org. Chem. 41 (1976) 722-725. |
| [17] | J. Kashyap, A.B. Chetry, P.J. Das, Synthesis of 4-arylidene-2-phenyloxazol-5-ones using 1:1 mixture of Al2O3-H3BO3, Synth. Commun. 28 (1998) 4178-4191. |
| [18] | F.M. Bautista, J.M. Campelo, A. García, et al., Study on dry-media microwave azalactone synthesis on different supported KF catalysts: influence of textural and acid-base properties of supports, J. Chem. Soc., Perkin Trans. 2 (2002) 227- 234. |
| [19] | K.A. Monk, D. Sarapa, R.S. Mohan, Bismuth (III) acetate: a new catalyst for preparation of azlactones via the Erlenmeyer synthesis, Synth. Commun. 30 (2000) 3167-3170. |
| [20] | M.M. Khodaei, A.R. Khosropour, S.J.H. Jomor, Efficient and chemoselective conversion of aryl aldehydes to their azalactones catalysed by Bi(III) salts under solvent free conditions, J. Chem. Res. Synop. (2003) 638-641. |
| [21] | P.S. Rao, R.V. Venkataratnam, Anhydrous zinc chloride catalyzed synthesis of 2- phenyl-4-arylidene-5(4H)-oxazolones, Indian J. Chem. Sect. 33B (10) (1994) 984- 985. |
| [22] | C. Yu, B. Zhou, W. Su, Z. Xu, Erlenmeyer synthesis for azlactones catalyzed by ytterbium(III) triflate under solvent-free conditions, Synth. Commun. 36 (2006) 3447-3453. |
| [23] | P.A. Conway, K. Devine, F. Paradisi, A simple and efficient method for the synthesis of Erlenmeyer azlactones, Tetrahedron 65 (2009) 2935-2938. |
| [24] | A.R. Khosropour, M.M. Khodaei, S.J. Hoseini Jomor, A new, efficient and chemoselective one-pot protocol for synthesis of 4-arylidene-2-phenyl-5(4H)-oxazolones from aryl aldehyde bisulfite adducts promoted by POCl3, J. Heterocycl. Chem. 45 (2008) 683-686. |
| [25] | M. Rostami, A.R. Khosropour, V. Mirkhani, et al., [C6(MIm)2]2W10O32. 2H2O:a novel and powerful catalyst for the synthesis of 4-arylidene-2-phenyl-5(4)- oxazolones under ultrasonic condition, C. R. Chim. 14 (2011) 869-877. |
| [26] | B. Samani Ghaleh Taki, V. Mirkhani, I. Mohammadpoor-Baltork, et al., Synthesis and characterization of nano silica supported tungstophosphoric acid: an efficient, reusable heterogeneous catalyst for the synthesis of azlactones, J. Inorg. Organomet. Polym. 23 (2013) 758-765. |
| [27] | H. Moghanian, M. Shabanian, H. Jafari, Microwave-assisted efficient synthesis of azlactone derivatives using TsCl/DMF under solvent-free conditions, C. R. Chim. 15 (2012) 346-349. |
| [28] | O. Lanitou, D. Dimotikali, E. Yannakopoulou, K. Papadopoulos, Studies on the catalytic activity of novel hybridized chiral organo-inorganic catalysts for epoxidation and alkylation reactions, Chem. Eng. J. 134 (2007) 72-77. |
| [29] | H. Paul, S. Basu, S. Bhaduri, G.K. Lahiri, Platinum carbonyl derived catalysts on inorganic and organic supports: a comparative study, J. Organomet. Chem. 689 (2004) 309-316. |
| [30] | K. Motokura, N. Viswanadham, G. Murali Dhar, Y. Iwasawa, Creation of acid-base bifunctional catalysis for efficient C-C coupling reactions by amines immobilization on SiO2, silica-alumina, and nano-H-ZSM-5, Catal. Today 141 (2009) 19-24. |
| [31] | S. Huh, H.T. Chen, J.W. Wiench, M. Pruski, V.S.Y. Lin, Cooperative catalysis by general acid and base bifunctionalized mesoporous silica nanospheres, Angew. Chem. Int. Ed. 44 (2005) 1826-1830. |
| [32] | X.Y. Shi, J.F. Wei, Selective oxidation of sulfide catalyzed by peroxotungstate immobilized on ionic liquid-modified silica with aqueous hydrogen peroxide, J. Mol. Catal. A: Chem. 280 (2008) 142-147. |
| [33] | D. Brunel, Functionalized micelle-templated silicas (MTS) and their use as catalysts for fine chemicals, Microporous Mesoporous Mater. 27 (1999) 329- 344. |
| [34] | M.E. Chimienti, L.R. Pizzio, C.V. Cáceres, M.N. Blanco, Tungstophosphoric and tungstosilicic acids on carbon as acidic catalysts, Appl. Catal. A: Gen. 208 (2001) 7-19. |
| [35] | Y. Kamiya, T. Okuhara, M. Misono, et al., Catalytic chemistry of supported heteropolyacids and their applications as solid acids to industrial processes, Catal. Surv. Asia 12 (2008) 101-113. |
| [36] | V.M. Joshi, R.L. Magar, P.B. Throat, et al., Novel one-pot synthesis of 4H-chromene derivatives using amino functionalized silica gel catalyst, Chin. Chem. Lett. 25 (2014) 455-458. |
| [37] | X. Shen, Y. Zhai, Y. Sun, H. Gu, Preparation of monodisperse spherical SiO2 by microwave hydro-thermal method and kinetics of dehydrated hydroxyl, J. Mater. Sci. Technol. 26 (2010) 711-714. |
| [38] | A. Saffar-Teluri, Boron trifluoride supported on nano-SiO2: an efficient and reusable heterogeneous catalyst for the synthesis of bis(indolyl)methanes and oxindole derivatives, Res. Chem. Intermed. 40 (2014) 1061-1067. |
| [39] | J. Safaei-Ghomi, R. Teymuri, H. Shahbazi-Alavi, A. Ziarati, SnCl2/nano SiO2: a green and reusable heterogeneous catalyst for the synthesis of polyfunctionalized 4Hpyrans, Chin. Chem. Lett. 24 (2013) 921-925. |
| [40] | B.F. Mirjalili, A. Bamoniri, M.A. Mirhoseini, Nano-SnCl4·SiO2 - a versatile and efficient catalyst for synthesis of 14-aryl- or 14-alkyl-14H-dibenzo[a,j]xanthenes, Chem. Heterocycl. Compd. 48 (2012) 856-860. |
| [41] | Q. Zhang, Z. Ye, S.T. Wang, J. Yin, Facile one-pot synthesis of PEGylated monodisperse mesoporous silica nanoparticles with controllable particle sizes, Chin. Chem. Lett. 25 (2014) 257-260. |
| [42] | N. Foroughifar, A. Mobinikhaledi, H. Moghanian, A straightforward and efficient catalyst-free one-pot synthesisof N-acyl-1, 3-diaryl-2-azaphenalene derivatives via multicomponent reactions, Chem. Lett. 39 (2010) 180-181. |
| [43] | A. Mobinikhaledi, H. Moghanian, M. Deinavizadeh, pTSA-catalyzed condensation of xylenols and aldehydes under solvent-free conditions: one-pot synthesis of 9H-xanthene or bisphenol derivatives, C. R. Chim. 16 (2013) 1035-1041. |
| [44] | H. Moghanian, A. Mobinikhaledi, A.G. Blackman, E. Sarough-Farahani, Sulfanilic acid-functionalized silica-coated magnetite nanoparticles as an efficient, reusable and magnetically separable catalyst for the solvent-free synthesis of 1-amidoand 1-aminoalkyl-2-naphthols, RSC Adv. 4 (2014) 28176-28185. |
| [45] | M.A. Nasseri, M. Sadeghzadeh, Multi-component reaction on free nano-SiO2 catalyst: excellent reactivity combined with facile catalyst recovery and recyclability, J. Chem. Sci. 125 (2013) 537-544. |
| [46] | T. Zeng, L. Yang, R. Hudson, et al., Fe3O4 nanoparticle-supported copper(I) pybox catalyst: magnetically recoverable catalyst for enantioselective direct-addition of terminal alkynes to imines, Org. Lett. 13 (2011) 442-445. |
| [47] | F. Adam, K. Mohammed Hello, H. Osman, Esterification via saccharine mediated silica solid catalyst, Appl. Catal. A: Gen. 365 (2009) 165-172. |
| [48] | K.A. Yeboah, J.D. Boyd, K.A. Kyeremateng, et al., Large accelerations from small thermal differences: case studies and conventional reproduction of microwave effects on palladium couplings, Reac. Kinet. Mech. Cat. 112 (2014) 295-304. |
| [49] | M. Rostami, A. Khosropour, V. Mirkhani, et al., Organic-inorganic hybrid polyoxometalates: efficient, heterogeneous and reusable catalysts for solvent-free synthesis of azlactones, Appl. Catal. A: Gen. 397 (2011) 27-34. |
| [50] | B.R. Madje, M.B. Ubale, J.V. Bharad, M.S. Shingare, Alum an efficient catalyst for Erlenmeyer synthesis, S. Afr. J. Chem. 63 (2010) 158-161. |
| [51] | H.C. Song, Y.F. Sun, W.M. Li, et al., Second nonlinear polarizability of 4-substituted- benzylideneoxazol-5(4H)-ones and 9-substituted-phenylacridines, Acta Chim. Sinica 59 (2001) 1563-1565. |
| [52] | S.G. Patil, R.R. Bagul, V.M. Kamble, V.A. Navale, A green protocol for Erlenmeyer Plö chl reaction by using [bmIm]OH, J. Chem. Pharm. Res. 3 (2011) 285-290. |
| [53] | J.D. Fissekis, C.G. Skinner, W. Shive, Synthesis and biological activity of some cycloalkaneglyoxylic acids, J. Am. Chem. Soc. 81 (1959) 2715-2718. |
| [54] | T. Cleary, J. Brice, N. Kennedy, F. Chavez, One-pot process to Z-a-benzoylaminoacrylic acid methyl esters via potassium phosphate-catalyzed Erlenmeyer reaction, Tetrahedron Lett. 51 (2010) 625-628. |
| [55] | S. Paul, P. Nanda, R. Gupta, A. Loupy, Ac2O-Py/basic alumina as a versatile reagent for acetylations in solvent-free conditions under microwave irradiation, Tetrahedron Lett. 43 (2002) 4261-4265. |
| [56] | I.C. Ivanov, T.N. Glasnov, D. Heber, Synthesis of 2H-chromeno[4,3-b]pyridine- 2,5(1H)-diones and related heterocycles via the Erlenmeyer-Ploechl reaction, J. Heterocycl. Chem. 42 (2005) 857-861. |
| [57] | G. Romanelli, J.C. Autino, P. Va’zquez, et al., A suitable synthesis of azlactones (4- benzylidene-2-phenyloxazolin-5-ones and 4-alkylidene-2-phenyloxazolin-5- ones) catalyzed by silica-alumina supported heteropolyacids, Appl. Catal. A: Gen. 352 (2009) 208-213." |