b Medical College of Jiaying University, Meizhou 514031, China
The asialoglycoprotein receptor (ASGPR) is a high-capacity Ctype lectin receptor expressed on mammalian hepatocytes,which plays an important role in the lysosomal processing of Nacetylgalactosamine (GalNAc) and galactose-containing glycopeptide substrates [1]. These motifs can therefore be designed as hepatotropic vectors for hepatocytes uptake of a variety of nanoparticles such as cyclodextrin [2],liposomes [3, 4] and polymers linked to oligonucleotide [5].
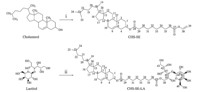
In this paper,a novel glycolipid,(5-cholesten-yl)[(4-O-b-Dgalactopyranosyl)-D-glucitol-6-yl]sebacate (CHS-SE-LA),was designed and synthesized. The glycolipid included three parts: homing device,spacer and anchor. Comprising a galactosyl residue,lactitol is usually used as a recognition moiety for the hepatocyte-targeting carrier [6]. Cholesterol,one of the lipid components used to form liposomes,is usually selected as the lipophilic anchor moiety for stably introducing the galactosyl moiety into liposome [7, 8, 9, 10]. Vinyl esters as acyl donors in many ways was the first choice in lipase catalyzed transesterification reactions [11],and consequently divinyl sebacate was chosen as a spacer part of link between cholesterol and lactitol.
There are various methods to attach ligands to the liposomes. For example,the ligand was coupled with long-chain fatty acids or hydrophobic phospholipid,then reacted with a drug loaded liposomes and formed a ligand-modified liposomes. However, most reactions are chemical reactions [7, 12, 10, 13, 9, 14, 15, 16],and these chemical modifications require multistep reactions,complicated and controlled reactions conditions and show extremely low regioselectivity for the esterification of sugar. An enzyme method was sought to solve these problems. The enzymatic reaction has advantages in liposomes surface modification,such as high initial rate,high substrate conversion,high regioselectivity,environmental protection and a reduction in energy consumption. So,in this work,we investigated a facile synthesis method of galactosyl ligandsviaenzymatic coupling method,constructed galactose-modified liposomes and tested the potential of galactosylated liposomes for hepatoma targeting. Our interest in enzymatic chemoselective esterification was its application to the synthesis of biomaterials exhibiting strong binding to specific lectins,which was essential for its application as a scaffold and drug delivery system with recognition ability to cells. 2. Experimental
The synthesis of (5-cholesten-yl)vinyl decanedioic acid (CHSSE): Cholesterol (0.13 mmol),divinyl sebacate (1.16 mmol), Candida rugosa lipase (CRL,38.5 mg,628 U/g,Sigma-Aldrich) and isooctane (5 mL) were added into a 10 mL screw-capped vial. The vial was placed in an air-bath at 35℃ and the reaction mixtures were shaken at 250 rpm for 11.5 h (Scheme 1),then the lipase was removed by suction filtration and the filtrate was evaporated to dryness. 250 mL methanol was added to the residue, then the mixture was dissolved and cooled at 0℃ overnight,then the precipitated white crystals were filtered,yielding 0.357 g (0.12 mmol,92.8%) CHS-SE.
|
Download:
|
| Scheme 1.The synthesis of galactosyl ligandviaenzymatic esterification of sugar alcohol. Conditions: (i) divinyl sebacate,isooctane,35℃,Candida rugosalipase; (ii) CHS-SE, pyridine: acetone (2:1,v:v),55℃,Novozym 435. | |
The synthesis of CHS-SE-LA: Lactitol (0.04 mmol),CHS-SE (0.15 mmol),Novozym 435 lipase (CAL-B,22.8 mg,798 U/g, Novozymes A/S Co),pyridine: acetone (2:1,v:v) 2.1 mL were added into a 10 mL screw-capped vial. It was placed in an air-bath at 55℃ and the reaction mixtures were shaken at 250 rpm for 31.1 h (Scheme 1). Then the lipase was removed by suction filtration and the filtrate was evaporated to dryness. The residue was washed with 50 mL cold isooctane to remove residual CHS-SE,and then the residue was lyophilized yielding 0.034 g CHS-SE-LA,which was a novel compound (0.038 mmol,94.3%). Its structure was determined by the aid of ESI-MS, 1H NMR and 13C NMR.
1-Palmitoyl-2-[12-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)aminododecanoyl]-sn-glycero-3-phosphocholine (NBD),as a fluorescence probe,was encapsulated in conventional liposomes (FL) and galactosylated liposomes (the mass ratios of CHS-SE-LA/lipid was 1:9,GAL-FL). The cell binding and internalization of NBD labeled liposomes were also studied just as Minyan Wei described [17]. In brief,HepG2 cells were seeded onto 24-well plates with 5×105 cells per well and cultured for 24 h. Then cells were incubated for 1 h at 37℃ with FL and GAL-FL,respectively,in which the final concentration of lipids (incorporated with 1%,m/m NBD) was 1 mmol/mL. For the inhibition study,1 mg/mL lactose was added to the emulsion solution in advance. After incubation, the cells were washed three times with 1 mL of PBS (pH 7.4),then were solubilized in 1% of TriotnX-100 PBS. The fluorescence intensity was assayed using a microplate reader (Flex Station3, Molecular Devices,USA) at wavelength of 465 nm.
Statistical significance was determined by Student’st-test with p<0.05 indicating significant difference using SPSS 22.0 software (SPSS Inc.,Chicago,IL,USA). 3. Results and discussion
Under mild conditions,CHS-SE and CHS-SE-LA are synthesized succinctly and efficiently by enzymatic method. The purified CHSSE or CHS-SE-LA was analyzed by MS and NMR,respectively. A mass spectrum was obtained by Mass Spectrometry (Thermo LCQ FLEET CA,USA) with positive ESI mode. 1H NMR and 13C NMR spectra were measured in CDCl3 for CHS-SE or pyridine-d5 for CHS-SE-LA,with a Bruker NMR spectrometer (Avance III Switzerland),respectively. The data is shown below:
CHS-SE: ESI-MS m/z: 619.51 [M+Na]+ . 13C NMR (125 MHz, CDCl3):δ173.57 (C-28),171.15 (C-37),141.50 (C-38),140.02 (C-6), 122.90 (C-9),97.76 (C-39),74.01 (C-2),56.99 (C-14),56.43 (C-15),50.32 (C-7),42.61 (C-13),40.03 (C-12),39.82 (C-22), 38.46 (C-4),37.30 (C-3),36.90 (C-5),36.48 (C-20),36.09 (C-18), 34.97 (C-29),34.22 (C-36),32.21 (C-10),32.16 (C-8),29.34 (C-31), 29.32 (C-32,C-33),29.26 (C-34),28.53 (C-16),28.32 (C-23),28.12 (C-1),25.30 (C-30),24.85 (C-35),24.58 (C-17),24.13 (C-21),23.12 (C-25),22.86 (C-24),21.33 (C-11),19.63 (C-27),19.01 (C-19),12.16 (C-26). 1H NMR (500 MHz,CDCl3): d7.28 (m,1 H),5.37 (d,1 H, J=4.5 Hz),4.87 (dd,1 H,J=14.0,1.5 Hz),4.65-4.53 (m,2 H),2.38 (t,2 H,J=7.5 Hz),2.35-2.22 (m,4 H),2.06-1.93 (m,2 H),1.93-1.81 (m,3 H),1.81-1.58 (m,5 H),1.58-1.22 (m,20 H),1.22-0.83 (m,23 H).
CHS-SE-LA: ESI-MS m/z: 919.50 [M+Na]+ . 13C NMR (100 MHz, pyridine-d5):δ173.45 (C-28),172.80 (C-37),139.81 (C-6),122.55 (C-9),106.23 (C-44),84.68 (C-40),76.80 (C-48),74.96 (C-46),73.61 (C-2),72.74 (C-42),72.67 (C-45),71.46 (C-39),70.15 (C-41),69.62 (C-47),66.50 (C-38),63.63 (C-43),61.59 (C-49),56.55 (C-14),56.13 (C-15),50.02 (C-7),42.25 (C-4),39.70 (C-13),39.49 (C-12),38.36 (C-22),37.01 (C-3),36.58 (C-5),36.25 (C-20),35.78 (C-18),34.50 (C-29),34.12 (C-36),31.90 (C-10),31.80 (C-8),29.08 (C-31,C-34), 29.03 (C-32,C-33),28.25 (C-1),27.98 (C-16),27.95 (C-23),25.10 (C-30),24.93 (C-35),24.24 (C-17),23.90 (C-21),22.68 (C-25),22.44 (C-24),21.03 (C-11),19.13 (C-27),18.71 (C-19),11.75 (C-26). 1H NMR (400 MHz,pyridine-d5):δ5.42 (d,1H,J=4.2 Hz),5.15 (d,1H, J=7.8 Hz),4.91-4.82 (m,2H),4.81-4.62 (m,4H),4.59 (t,1H, J=5.4 Hz),4.54-4.28 (m,7H),4.10 (dd,1H,J=9.4,3.3 Hz),4.04 (t, 1H,J=6.2 Hz),2.59-2.44 (m,2H),2.35 (dt,4H,J=33.7,7.5 Hz), 2.06-1.78 (m,4H),1.75-1.34 (m,14H),1.33-1.02 (m,20H),0.99 (d, 3H,J=6.4 Hz),0.91 (d,6H,J=6.6 Hz),0.69 (s,3H).
There are eight hydroxyl groups in lactitol,thereby requiring chemoselective esterification prior to use as monomers. After resultant compound was purified by the column chromatography, the chemoselectivity was confirmed by 13C NMR,in which the chemical shifts of esterified carbons have been reported to show a downfield shift [6, 18]. In the 13C NMR of lactitol,three peaks were observed atd 61.4,63.3,and 63.8 due to the primary hydroxymethylenes at the 6' ,1,an δ 6 positions,respectively. In the 13C NMR of the resultant product with CAL-B,the methylene group at the C-6 position alone was shifted to a lower magnetic field (δ 66.5),about 3 ppm shift. Meanwhile neighboring C-5 position was shifted to a higher magnetic field about 1 ppm indicating that the chemoselective esterification proceeded (Fig. 1). Furthermore we proposed here the use of HMBC as an experiment for rapid chemical shift assignment of the esterified lactitol by lipase CAL-B. The HMBC correlations between H-6a (δH4.77)/H-6b (δH4.87) and C-37 (δc 172.80) further confirmed that the esterification of lactitol only occurs at C-6 position (Fig. 2). The enzymatic estrification of polyol was reported to be affected by the steric hindrance around the hydroxyl group, and the substitutions of the primary and secondary alcohols are remarkably discriminated [19]. The hydroxyl groups of lactitol at C1,C6and C'6 are highly reactive with lipases. In the case of lactitol,CAL-B showed an outstanding selectivity to the hydroxyl group at the C6 position,but not to those at the C'6or C1 positions.

|
Download:
|
| Fig. 1. Expanded 13C NMR of the esterified lactitol by lipase CAL-B (top),and lactitol (bottom). | |

|
Download:
|
| Fig. 2. 500 MHz HMBC spectrum of the esterified lactitol by lipase CAL-B in pyridine-d5 | |
The cell association fluorescence of GAL-FL by ASGPR overexpressed HepG2 cells was as much as 2.6-fold (p<0.01) FL (Fig. 3). Meanwhile the cell association of GAL-FL was inhibited by preincubation with 1 mg/mL of excessive lactose (p<0.01) in HepG2 cells,and FL controls group showed no significant differences. It was suggested that CHS-SE-LA could enhance the cell binding and internalization of GAL-FL by a specific receptorendocytosis mechanism in HepG2 cells.

|
Download:
|
| Fig. 3. Uptake of NBD-labeled GAL-FL or FL by HepG2 cells. Each value represents the mean±SD,n= 3. (**p<0.01). | |
In summary,we synthesized novel galactosylated cholesterol derivatives for developing a hepatocyte specific drug carrier by the using of enzyme catalysis. There are several reports about chemical synthesis of similar galactosylated cholesterol [10, 14, 20],but all of those synthesis methods are complicated,low yield and environmentally unfriendly. Compared with chemical synthesis,enzymatic synthesis shows high specificity,efficiency and hypotoxicity. In HepG2 cells,the liposomes containing galactosylated cholesterol derivatives showed higher transfection activities than controls liposomes based on a receptor-mediated mechanism. These results suggested that the liposomes containing CHS-SE-LA could be a potential drug delivery carrier for hepatocyte-selective targeting.
AcknowledgmentsThis work was financially supported by the Ph.D. Programs Foundation of Ministry of Education of China (No. 20134425110010) and the Special Funds from Central Finance of China in Support of Local Colleges and University [No. 276(2014)].
| [1] | A.N. Zelensky, J.E. Gready, The C-type lectin-like domain superfamily, FEBS J. 272 (2005) 6179-6217. |
| [2] | G.J. Bernardes, R. Kikkeri, M. Maglinao, et al., Design, synthesis and biological evaluation of carbohydrate-functionalized cyclodextrins and liposomes for hepatocyte- specific targeting, Org. Biomol. Chem. 8 (2010) 4987-4996. |
| [3] | S.L. Wang, F.B. Yu, T.Y. Jiang, et al., Design and synthesis of novel galactosylated polymers for liposomes as gene drug carriers targeting the hepatic asialoglycoprotein receptor, J. Drug Target. 16 (2008) 233-242. |
| [4] | M. Yamamoto, K. Ichinose, N. Ishii, et al., Utility of liposomes coated with polysaccharide bearing 1-amino-lactose as targeting chemotherapy for AH66 hepatoma cells, Oncol. Rep. 7 (2000) 107-111. |
| [5] | D.B. Rozema, D.L. Lewis, D.H. Wakefield, et al., Dynamic polyConjugates for targeted in vivo delivery of siRNA to hepatocytes, Proc. Natl. Acad. Sci. U. S. A. 104 (2007) 12982-12987. |
| [6] | Y. Miura, T. Ikeda, K. Kobayashi, Chemoenzymatically synthesized glycoconjugate polymers, Biomacromolecules 4 (2003) 410-415. |
| [7] | L.A. Sliedregt, P.C. Rensen, E.T. Rump, et al., Design and synthesis of novel amphiphilic dendritic galactosides for selective targeting of liposomes to the hepatic asialoglycoprotein receptor, J. Med. Chem. 42 (1999) 609-618. |
| [8] | S. Kawakami, J. Wong, A. Sato, et al., Biodistribution characteristics of mannosylated, fucosylated, and galactosylated liposomes in mice, Biochim. Biophys. Acta 1524 (2000) 258-265. |
| [9] | P.C. Rensen, L.A. Sliedregt, M. Ferns, et al., Determination of the upper size limit for uptake and processing of ligands by the asialoglycoprotein receptor on hepatocytes in vitro and in vivo, J. Biol. Chem. 276 (2001) 37577-37584. |
| [10] | S.N. Wang, Y.H. Deng, H. Xu, et al., Synthesis of a novel galactosylated lipid and its application to the hepatocyte-selective targeting of liposomal doxorubicin, Eur. J. Pharm. Biopharm. 62 (2006) 32-38. |
| [11] | N. Turan, J.F. Kennedy, in: Munishwar Nath Gupta (Ed.), Methods in Non-aqueous Enzymology, 47, Birkhäuser Verlag, Basel, 2002, , x + 218 pp., sFr. 228, Carbohydrate Polymers 47 (2002) 88. |
| [12] | E.A. Biessen, D.M. Beuting, H.C. Roelen, et al., Synthesis of cluster galactosides with high affinity for the hepatic asialoglycoprotein receptor, J. Med. Chem. 38 (1995) 1538-1546. |
| [13] | R.T. Lee, M.H. Wang, W.J. Lin, Y.C. Lee, New and more efficient multivalent glycoligands for asialoglycoprotein receptor of mammalian hepatocytes, Bioorg. Med. Chem. 19 (2011) 2494-2500. |
| [14] | S. Kawakami, F. Yamashita, M. Nishikawa, Y. Takakura, M. Hashida, Asialoglycoprotein receptor-mediated gene transfer using novel galactosylated cationic liposomes, Biochem. Biophys. Res. Commun. 252 (1998) 78-83. |
| [15] | C. Managit, S. Kawakami, F. Yamashita, M. Hashida, Effect of galactose density on asialoglycoprotein receptor-mediated uptake of galactosylated liposomes, J. Pharm. Sci. 94 (2005) 2266-2275. |
| [16] | S. Kawakami, C. Munakata, S. Fumoto, F. Yamashita, M. Hashida, Novel galactosylated liposomes for hepatocyte-selective targeting of lipophilic drugs, J. Pharm. Sci. 90 (2001) 105-113. |
| [17] | M. Wei, Y. Xu, Q. Zou, et al., Hepatocellular carcinoma targeting effect of PEGylated liposomes modified with lactoferrin, Eur. J. Pharm. Sci. 46 (2012) 131-141. |
| [18] | J. Chopineau, F.D. McCafferty, M. Therisod, A.M. Klibanov, Production of biosurfactants from sugar alcohols and vegetable oils catalyzed by lipases in a nonaqueous medium, Biotechnol. Bioeng. 31 (1988) 208-214. |
| [19] | A. Halldorsson, C.D. Magnusson, G.G. Haraldsson, Chemoenzymatic synthesis of structured triacylglycerols, Tetrahedron Lett. 42 (2001) 7675-7677. |
| [20] | F. Yu, T. Jiang, J. Zhang, L. Cheng, S. Wang, Galactosylated liposomes as oligodeoxynucleotides carrier for hepatocyte-selective targeting, Pharmazie 62 (2007) 528-533. |




