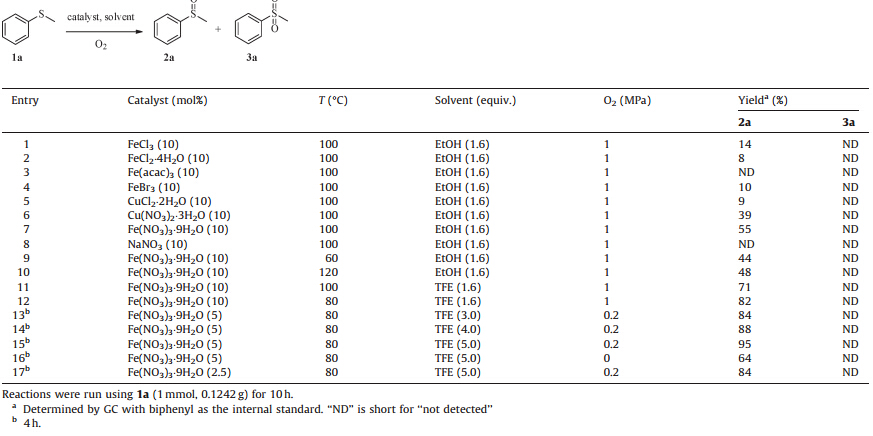
Sulfoxides,mainly as chiral auxiliaries frequently employed in asymmetric synthesis [1],have been widely applied in the preparation of biologically active molecules [2]. One of the most favored methods for the preparation of sulfoxides is based on the sulfides oxidation as shown in Scheme 1 [3]. A large number of metal catalysts have been developed for this transformation,such as V [4],Mn [5],Os [6],Sc [7],Ti [8],Re [9],Co [10],Cr [11],W [12], Cu [13]. Iron catalysts,with the advantages of environmentally benign,easily available,low toxic and inexpensive,have also been used for this purpose,such as Fe(acac)2/PEG [14],Fe(acac)3/Schiff base [15],and Fe(Salen) [16] systems. However,most of the ironcatalyzed processes commonly require complicated ligands or toxic solvent and thus could pose extra environment burdens. Moreover,selective sulfoxidation is still a major challenge as most reported protocols involving overoxidation to sulfones. Consequently,it is desirable to develop more environmentally benign processes for the oxidation of sulfides with high efficiency and selectivity.
|
Download:
|
| Scheme 1.Oxidation of sulfides | |
Oxygen,being abundant,inexpensive,and easily available, could be an ideal oxidant with water as the sole byproduct,which is in line with the criterion of green chemistry. On the other hand, nitrate anion is found to be free or coordinated to a metallic center and the reactivity depends on the nature of the metal-nitrate bond. Besides,nitrate anion shows a complicated oxidation-reduction mechanism that involves various oxidation states of the nitrogen [17]. Herein,we wish to report an inexpensive Fe(NO2)3·9H2Opromoted oxidation of sulfides with O2as the oxidant,resulting in high yield and excellent selectivity of the corresponding sulfoxides. 2. Experimental
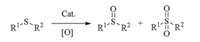
We began our research by using thioanisole (1a) as a model substrate under 1 MPa of oxygen. The results are summarized in Table 1. Initially,small amounts of phenylmethyl sulfoxide was obtained when FeCl3,FeCl2·4H2O,Fe(acac)3 and FeBr3 were selected respectively as a catalyst,and nearly no sulfone was detected (entries 1-4,Table 1). To be delighted,the highest reactivity groups were given through the system of Fe(NO2)3·9H2O and Cu(NO3)2·3H2O (entries 6 and 7,Table 1),implying that nitrate anion plays an important role in promoting the reaction. In addition,NaNO3alone was demonstrated to be ineffective (entry 8),in agreement with the previous report [18]. Consequently, Fe(NO2)3·9H2O was selected as the catalyst for further investigation. Reaction temperature could also play an important role in the reaction outcome. Indeed,the highest yield was attained at 100℃, while higher or lower temperature would have negative effect on the reaction (entries 7,9,and 10). On the other hand,when TFE was chosen as the solvent,a higher yield was obtained in comparison with EtOH (entry 11). This is understandable because the acidity of TFE is strong than EtOH (pKa(EtOH)= 15.2,pKa(TFE)= 12.4). TFE has been used as an ideal polar solvent for the oxidation of sulfides to the corresponding sulfoxides with the advantage of non-nucleophile and non-hydrogen bonding acceptor [19]. Accordingly,TFE was chosen as the suitable solvent. When the temperature was decreased to 80℃,82% of sulfoxide was achieved (entry 12). Raising the amount of TFE,high yield still was preserved even lowering the amount of Fe(NO2)3·9H2O (5 mol%) and the pressure of O2(0.2 MPa),and shorten the reaction time (4 h) (entry 13vs. 12). Sulfoxide yield was improved rapidly with increasing the TFE amount (entries 13-15). Excellent yield (95%) of sulfoxide was obtained when 5 equivalents of TFE were employed (entry 15). Without O2,64% of sulfoxides were afforded because there was about 0.4 mmol O2 in the autoclave from the air (entry 16). Subsequently,decreasing the amount of Fe(NO2)3·9H2O,the yield of sulfoxide reduced accordingly (entry 17).
| Table 1 Metal-promoted aerobic oxidation of thioanisole to phenylmethyl sulfoxide. |
Having established the suitable protocol,a series of sulfides were evaluated by performing the reaction under the given conditions [Fe(NO2)3·9H2O (5 mol%),O2(0.2 MPa),80℃,4 h,TFE (5.0 equiv.)] as summarized in Table 2. A wide variety of sulfides with electron-withdrawing or electron-donating group atparaposition,such as -Cl,-CH3and -OCH3,could undergo smoothly and gave good yields of the corresponding sulfoxides (entries 1-4, Table 2). However,a strong electron-withdrawing group (-CN) would lead to a slight low yield (entry 5). The reason seems to be that electron deficiency could result in the passivation of the substrate in the oxidation reaction. Interestingly,the oxidized product 2f referred to two functional groups (-CH2OH to -CHO, sulfide to sulfoxide) was generated when 4-(methylthio)-benzyl alcohol (1f) was selected as the substrate,indicating the potential strong oxidation characteristic involving in the oxidative system (entry 6). To obtain the higher yield,longer reaction time was required for diphenyl sulfide (1g) (entry 7). In the case of benzylphenyl sulfide (1h),only 60% yield of sulfoxide can be gained with low selectivity,and unknown compounds were observed probably due to decomposition of benzylphenyl sulfide (entry 8). In addition,di-n-butyl sulfide showed higher active than that of aromatic ones (entry 9).
| Table 2 Fe(NO2)3·9H2O-catalyzed oxidation of various sulfides in TFE |
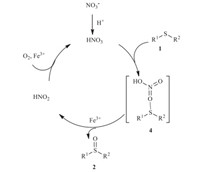
The acidity of TFE (pKa(TFE)= 12.4) [19] allows TFE to provide protons to the NO3- anion,with the formation of small amount HNO3 analogical species. It has been reported that HNO3could oxidize sulfides [17]. On the other hand,due to the limited small amount of HNO3 analog,the oxidation capacity of HNO3 is relatively weak. In this context,on the basis of the experimental results and the published reaction pathway,a plausible mechanism for the Fe(NO2)3·9H2O-catalyzed selective oxidation of sulfides to sulfoxides was proposed as depicted in Scheme 2. With the effect of acidic TFE,the HNO3analog species is firstly in situ formed. Subsequently,the sulfide is oxidized by HNO3through the formation of the intermediate 4,and the sulfoxide 2is formed with the release of hydrogen nitrite promoted by Fe3+ . Finally, hydrogen nitrate is regenerated by the oxidation of HNO2in the presence of oxygen and Fe3+ [18]. The limited amount of HNO3 shows weak capacity of oxidation,which could not further oxidize the sulfoxide2to the sulfone.
|
Download:
|
| Scheme 2.Proposed mechanism for the Fe(NO2)3·9H2O-catalyzed selective oxidation of sulfides to sulfoxides | |
In summary,we have developed an efficient Fe(NO2)3·9H2Ocatalyzed oxidation of sulfides with oxygen as the sole oxidant in TFE,leading to the exclusive formation of sulfoxides. In particular, the nitrate anion is believed to contribute to a complicated oxidation-reduction mechanism due to various oxidation states of the nitrogen under acidic conditions. This environmentally benign protocol affords sulfoxides in up to almost quantitative yields,and benzyl alcohol can also be oxidized to benzaldehyde,thus demonstrating great potential application in terms of green process and technological innovation.
AcknowledgmentsWe are grateful to the National Natural Science Foundation of China,Specialized Research Fund for the Doctoral Program of Higher Education (No. 20130031110013),MOE Innovation Team (No. IRT13022) of China for financial support.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.12.010.
| [1] | I. Fernández, N. Khiar, Recent developments in the synthesis and utilization of chiral sulfoxides, Chem. Rev. 103 (2003) 3651-3706. |
| [2] | E. Wojaczynska, J. Wojaczynski, Enantioselective synthesis of sulfoxides: 2000- 2009, Chem. Rev. 110 (2010) 4303-4356. |
| [3] | (a) J. Legros, J.R. Dehli, C. Bolm, Applications of catalytic asymmetric sulfide oxidations to the syntheses of biologically active sulfoxides, Adv. Synth. Catal. 347 (2005) 19-31; |
| [4] | S.L. Jain, B.S. Rana, B. Singh, et al., An improved high yielding immobilization of vanadium Schiff base complexes on mesoporous silica via azide-alkyne cycloaddition for the oxidation of sulfides, Green Chem. 12 (2010) 374-377. |
| [5] | (a) M. Hirano, S. Yakabe, J.H. Clark, K. Hiroyuki, M. Takashi, Manganese(III)- catalysed oxidation of sulphides with sodium chlorite in an aprotic solvent in the presence of alumina, Synth. Commun. 26 (1996) 1875-1886; |
| [6] | B.M. Choudary, C.R.V. Reddy, B.V. Prakash, M.L. Kantam, B. Sreedhar, The first example of direct oxidation of sulfides to sulfones by an osmate molecular oxygen system, Chem. Commun. 39 (2003) 754-755. |
| [7] | M. Matteucci, G. Bhalay, M. Bradley, Mild and highly chemoselective oxidation of thioethers mediated by Sc(OTf)3, Org. Lett. 5 (2003) 235-237. |
| [8] | M.A.M. Capozzi, C. Centrone, G. Fracchiolla, F. Nsao, C. Cardelliccio, A study of factors affecting enantioselectivity in the oxidation of aryl benzyl sulfides in the presence of chiral titanium catalysts, Eur. J. Org. Chem. 23 (2011) 4327-4334. |
| [9] | J.B. Arterburn, S.L. Nelson, Rhenium-catalyzed oxidation of sulfides with phenyl sulfoxide, J. Org. Chem. 61 (1996) 2260-2261. |
| [10] | P.J. Chai, Y.S. Li, C.X. Tan, An efficient and convenient method for preparation of disulfides from thiols using air as oxidant catalyzed by Co-salophen, Chin. Chem. Lett. 22 (2011) 1403-1406. |
| [11] | L. Xu, J. Cheng, M.L. Trudell, Chromium(VI) oxide catalyzed oxidation of sulfides to sulfones with periodic acid, J. Org. Chem. 68 (2003) 5388-5391. |
| [12] | A. Bordoloi, A. Vinu, S.B. Halligudi, One-step synthesis of SBA-15 containing tungsten oxide nanoclusters: a chemoselective catalyst for oxidation of sulfides to sulfoxides under ambient conditions, Chem. Commun. 45 (2007) 4806-4808. |
| [13] | I. Gamba, S. Palavicini, E. Monzana, L. Casella, Catalytic sulfoxidation by dinuclear copper complexes, Chem. Eur. J. 15 (2009) 12932-12936. |
| [14] | B. Li, A.H. Liu, L.N. He, et al., Iron-catalyzed selective oxidation of sulfides to sulfoxides with the polyethylene glycol/O2 system, Green Chem. 14 (2012) 130-135. |
| [15] | (a) J. Legros, C. Bolm, Iron-catalyzed asymmetric sulfide oxidation with aqueous hydrogen peroxide, Angew. Chem. Int. Ed. 42 (2003) 5487-5489; |
| [16] | (a) H. Egami, T. Katsuki, Fe(salan)-catalyzed asymmetric oxidation of sulfides with hydrogen peroxide in water, J. Am. Chem. Soc. 129 (2007) 8940-8941; |
| [17] | (a) O.W.J.S. Rutten, A. van Sandwijk, G. van Weert, The electrochemical reduction of nitrate in acidic nitrate solutions, J. Appl. Electrochem. 29 (1999) 87-92; |
| [18] | L.I. Rossi, S.E. Martín, Possible role of nitrate/nitrite redox cycles in catalytic and selective sulfoxidation reaction Metallic nitrates and bromides as redox mediators: a comparative study, Appl. Catal. A: Gen. 250 (2003) 271-278. |
| [19] | (a) I.A. Shuklov, N.V. Dubrovina, A. Bö rner, Fluorinated alcohols as solvents, cosolvents and additives in homogeneous catalysis, Synthesis 19 (2007) 2925-2943; |