b China Pharmaceutical University Pharmaceutical Co. Ltd., Nanjing 210009, China
Aminopeptidase is an important enzyme known as removing the NH2-terminal amino acid from protein and peptide substrates by catalyzing the hydrolysis of the N-terminal peptide bond. The enzyme is indispensable in the process of cell growth. One of the most important aminopeptidase members is aminopeptidase N (APN,EC 3.4.11.2),which is a zinc-dependent exopeptidase that expresses on the surface of different cells,such as epithelial cells of the small intestine and the proximal renal tubules,synaptic membranes in the central nervous system,as well as the fibroblasts,monocytes and myeloid progenitors [1, 2, 3, 4]. APN has revealed to be over-expressed on tumor cells,which plays a pivotal role in tumor invasion and diffusion by participating in the degradation of extracellular matrix [4, 5]. For this reason,several probes directed toward APN/CD13 have been developed for diagnostic and therapeutic propose [1, 6].
As for cell imaging,the recognition moiety linking to molecular markers is necessary for the sensitivity. So far,most of the reported APN probes utilize Asn-Gly-Arg peptide (NGR) as the recognition moieties [7]. NGR is a tripeptide that has been demonstrated to home specifically to APN on tumor endothelium [8, 9]. However, NGR has just a moderate binding affinity to APN/CD13 [10]. To achieve a high affinity probe for APN,the Gali’s research group synthesized a Tc-99m labeled Probestin (an APN inhibitor) conjugates. Nevertheless,such a radioisotope is harmful to patients and practitioners [11]. By contrast,small-molecule fluorescent probe is relatively inexpensive and has no radioactive spill. What’s more,fluorescent probe is sensitive equal to that of radionuclides labels and can be easily and quickly detected by relatively simple and inexpensive instruments [12, 13, 14]. Therefore, in this article,we undertake an effort to branch out a novel Bestatin-based fluorescent probe (Bes-Green,2) with high affinity to APN and potential in APN cellular imaging. 2. Experimental 2.1. Synthesis of Bes-Green
The synthesis of Bes-Green (2) is shown in Scheme 1. The details for preparation and NMR and HRMS spectra of this molecule are presented in supporting information.
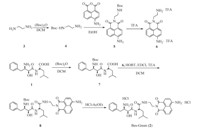
| Download: |
| Scheme 1. The synthesis of Bes-Green. | |
The docking studies were performed using the Sufλex-Dock docking program in Sybyl-X 1.2 (Tripos Inc.,St. Louis,MO,USA) software running on a Dell Precision T5500 workstation. The molecular structures were built based on the conformation of Bestatin. Energy minimization was performed using Powell optimization in the presence of the Tripos force field with a convergence criterion of 0.05 kcal/mol Å and then assigned with the Gasteiger-Hückel charges. In the docking process,maximum number of poses per ligand was set to 20 and other parameters were set as default. 2.3. Spectroscopic study
Solutions of Bes-Green were prepared in 0.1 mol/L Tris-HCl buffer at pH 7.5. Absorption spectra were recorded using Shimadzu UV-1700 UV-visible spectrometer. Fluorescence spectra were recorded using Varioskan (Thermo Electron Corporation). Quantum yield was calculated using fluorescein (Φ= 0.92 in 0.1 mol/L NaOH) as standard [15]. 2.4. IC50assay on enzyme level
IC50 of Bestatin on the enzymetic level against APN was determined using Ala-PABA-7HC (25mmol/L final concentration) as the substrates and porcine kidney aminopeptidase (BioCol GmbH) as the enzyme in aTris-HCl buffer (100 mmol/L,pH 7.5). The hydrolysis of the substrate was monitored by following the changes in the ratios of fluorescent intensities at 450 and 390 nm (λex= 330 nm). All solutions of the inhibitor were prepared in the assay buffer. All inhibitors were preincubated with APN for 30 min at 378C. The assay mixture,which contained the inhibitor solution (with its concentration from 0.205mmol/L to 210mmol/L),the enzyme solution (1 mIU/mL,final concentration),and the assay buffer,was adjusted to 200mL. IC50 values were calculated by nonlinear regression of at least five data points using SigmaPlot software. 2.5. Cell culture and fluorescent image assay
ES-2 cells were purchased from the Committee on Type Culture Collection of Chinese Academy of Sciences and grown in RPMI-1640 medium supplemented with 10% (v/v) fetal bovine serum in an atmosphere of 5% CO2,95% air at 37 8C. Cells were adjusted to the density of 20,000/well and incubated for 32 h,then washed with complete cell culture medium twice,and then incubated at 378C in the presence of Bes-Green (80mmol/L) or the mixture of Bes-Green (80mmol/L) and Bestatin (3.2 mmol/L) in RPMI-1640 medium for 2 h. Fluorescence imaging was performed using a Zeiss Axio Observer A1 fluorescence microscope. 3. Results and discussion
As reported by the WenFang Xu’s lab,Bestatin inhibits the activity of APN by tight binding of the active site [16]. In such a case,the aromatic ring and the aliphatic chain of the leucine residue can fit into the S1 and S1' pockets of APN by hydrophobic interactions; the 2-hydroxyl and the carbonyl groups can coordinates the catalytic zinc ion,and the protonated amino group can produce a salt bridge with Glu 355 near S1 pocket (Scheme 2). Moreover,the carboxyl group of Bestatin exhibited a minor effect on the inhibitory activity considering that BestatinCOOMe presented a similar IC50 to APN [16]. As a result,we attempt to tether Bestatin with a fluorophore at the carboxyl moiety (Scheme 1). In the current research,a 4-amino-1,8-naphthalimide moiety was preferred as the fluorophore in view of its beneficial optical properties,such as high photostability and large Stokes shift [17]. The docking results of Bestatin and BesGreen (Fig. 1A and B) suggested that such a probe could tightly bind to APN similarly to Bestatin. Besides,the 4-amino-1,8-naphthalic ring moiety was proposed to generate a cation-pinteraction with Arg 376 around the APN’s active site.

| Download: |
| Scheme 2. The design strategy of probe Bes-Green. | |
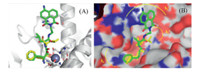
| Download: |
| Fig. 1. Docking results of Bestatin (yellow sticks) and Bes-Green(green sticks) into APN. (A) The crystal structure of APN (PDB Entry: 4FKK) was depicted in white ribbons,and the zinc ion was demonstrated in black sphere and (B) the stereo view of (A). | |
As depicted in Fig. 2,Bes-Green has a maximum absorption and emission peak at 450 and 550 nm in Tris-HCl buffer (pH 7.4), respectively. The Stokes shift (115 nm) is much larger than any current affinity-based APN probes [9, 18, 19, 20],which can substantially diminish the influence of excitation light in detections. Moreover,quantum yield of Bes-Green is 0.22 after careful examination. After pH titration,the fluorescent intensity of this probe demonstrates stable at pH>6 (Fig. S1 in Supporting information),however,the fluorescence has increasing tendency when pH decreases,which may be caused by the destroy of photoinduced electron transfer (PET) quenching [21].
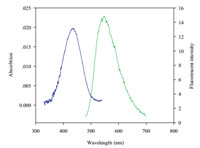
| Download: |
| Fig. 2. Absorption (blue) and emission (green) spectra of Bes-Green(20mmol/L) in Tris-HCl buffer (pH 7.4). | |
The inhibitory activity of Bes-Green and Bestatin was determined by using Ala-PABA-7HC as the substrate according to the literature [22]. As expected,Bes-Green demonstrated equivalent (even somewhat better) inhibitory potency to APN comparing with Bestatin (IC50=8versus22mmol/L). This result is another example to verify the fact that the carboxyl group of Bestatin displays a minor effect on the inhibitory activity. As mentioned in the docking result,the better inhibitory potency of Bes-Green maybe due to the cation-pinteraction between 4-amino-1,8-naphthalic moiety and Arg 376 around the APN’s active site. This evidence indicated that Bes-Green could bind to APN tightly,which is of importance for imaging assay. Considering that ES-2 cell lines derived from ovarian clear cell carcinoma with high expression of APN,to further confirm the practical feasibility of Bestatin-based probe, Bes-Green was applied for endogenous APN imaging in living ES-2 cells. As illustrated in Fig. 3,Bes-Green can light up ES-2 cells after 2 h incubation,and subsequently,such a fluorescent emission can be competitively quenched by 40-fold excess of an APN inhibitor, Bestatin.
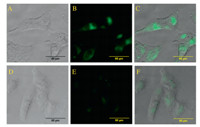
| Download: |
| Fig. 3. Fluorescence images of living ES-2 cells with Bes-Green: top row,ES-2 cells incubated with the Bes-Green for 2 h: (A) bright-field,(B) green channel,(C) merge of (A) and (B); bottom row,ES-2 cells incubated with the Bes-Green and Bestatin for 2 h: (D) bright-field,(E) green channel,(F) merge of (D) and (E). | |
Commonly,an inhibitor based fluorescent probe mainly contains three parts,the recognition moiety (inhibitor),the linker and the reporter (fluorophore). The first inhibitor-based fluorescent APN probe named Cy 5.5-23 for cell imaging was reported recently [23, 24]. This probe used NIR fluorophore Cy 5.5 as the reporter, which is excellent in deep tissue imaging. However,the recognition moiety (inhibitor) used in Cy 5.5-23 was not a potent APN ligand, which may lead to unpredicted and unspecific issue. So far,various inhibitors have been well developed toward APN,and one case in point is Bestatin,which is a dipeptide immunomodulator isolated from a culture filtrate of Streptomyces olivoreticuli with a skeleton of AHPA [25, 26]. As the sole marketed APN inhibitor,Bestatin’s pharmacokinetics and biotransformation has been examined in detail. A Bestatin-linker-fluorescein probe has been developed by the D.C. Greenbaum’s group [18],but they did not report the cellular APN imaging result. In current work,probe Bes-Green using a marketed drug as the recognition moiety showed good labeling ability to APN high expressed cells,and to the best of our knowledge,this is the first Bestatin-based fluorescent probe with high affinity to APN and efficiency of APN cellular imaging. We expect that these results may eventually lead to a brand new tool kit for biological study of cellular APN. Bes-Green contains a drug recognition moiety,and expected to be used in human tumor diagnosis. However,the detection wavelength of the report fluorophore is not high enough for deep tissue imaging. The design and studies of Bestatin-NIR fluorophore probes are ongoing in our research group and will be reported in the near future. 4. Conclusion
In summary,a Bestatin-based fluorescent probe (Bes-Green,2) was well designed and synthesized with reasonable optical properties such as large Stokes shift herein. After evaluation, Bes-Green demonstrated an equivalent inhibitory activity with Bestatin along with the capability of imaging endogenous APN in ES-2 living cells. These combined features of Bes-Green would be profitable in biological and medical research. Based on these interesting results,we are currently carrying out a structural modification and optimization of the APN-selective fluorescent probes that exhibit improved biological and optical characteristics.
Acknowledgments
The present work was supported by the Program for New Century Excellent Talents in University (No. NCET-11-0306),the Shandong Natural Science Foundation (No. JQ201019),the Independent Innovation Foundation of Shandong University, IIFSDU (No. 2014JC008) and the Graduate Independent Innovation Foundation of Shandong University,GIIFSDU (No. yzc12096).
Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.01.023.
| [1] | L.Z. Chen, W. Sun, J. Li, et al., The first ratiometric fluorescent probes for aminopeptidaseN cell imaging, Org. Biomol. Chem. 11 (2013) 378-382. |
| [2] | J. Dixon, L. Kaklamanis, H. Turley, et al., Expression of aminopeptidase-n (CD 13) innormal tissues and malignant neoplasms of epithelial and lymphoid origin, J. Clin.Pathol. 47 (1994) 43-47. |
| [3] | A.T. Look, R.A. Ashmun, L.H. Shapiro, S.C. Peiper, Human myeloid plasma membraneglycoprotein CD13 (gp150) is identical to aminopeptidase N, J. Clin. Invest.83 (1989) 1299-1307. |
| [4] | X.P. Zhang, W.F. Xu, Aminopeptidase N (APN/CD13) as a target for anti-canceragent design, Curr. Med. Chem. 15 (2008) 2850-2865. |
| [5] | I. Saiki, J. Yoneda, I. Azuma, et al., Role of aminopeptidase N (CD13) in tumor-cellinvasion and extracellular matrix degradation, Int. J. Cancer 54 (1993) 137-143. |
| [6] | H. Tsukamoto, K. Shibata, H. Kajiyama, et al., Aminopeptidase N (APN)/CD13inhibitor, Ubenimex, enhances radiation sensitivity in human cervical cancer,BMC Cancer 8 (2008) 74. |
| [7] | R. Pasqualini, E. Koivunen, R. Kain, et al., Aminopeptidase N is a receptor fortumor-homing peptides and a target for inhibiting angiogenesis, Cancer Res. 60(2000) 722-727. |
| [8] | F. Curnis, A. Sacchi, L. Borgna, et al., Enhancement of tumor necrosis factor alphaantitumor immunotherapeutic properties by targeted delivery to aminopeptidaseN (CD13), Nat. Biotechnol. 18 (2000) 1185-1190. |
| [9] | A.H. Negussie, J.L. Miller, G. Reddy, et al., Synthesis and in vitro evaluation of cyclicNGR peptide targeted thermally sensitive liposome, J. Control. Release 143 (2010)265-273. |
| [10] | L.A. Plesniak, B. Salzameda, H. Hinderberger, et al., Structure and activity ofCPNGRC: a modified CD13/APN peptidic homing motif, Chem. Biol. Drug Des.75 (2010) 551-562. |
| [11] | G. Pathuri, A.F. Hedrick, B.C. Disch, et al., Synthesis and evaluation of novel Tc-99m labeled probestin conjugates for imaging APN/CD13 expression in vivo,Bioconjug. Chem. 23 (2012) 115-124. |
| [12] | S. Lee, J. Xie, X. Chen, Peptides and peptide hormones for molecular imaging anddisease diagnosis, Chem. Rev. 110 (2010) 3087-3111. |
| [13] | H. Kobayashi, M. Ogawa, R. Alford, P.L. Choyke, Y. Urano, New strategies forfluorescent probe design in medical diagnostic imaging, Chem. Rev. 110 (2010)2620-2640. |
| [14] | E. Soini, I. Hemmilä, Fluoroimmunoassay: present status and key problems, Clin.Chem. 25 (1979) 353-361. |
| [15] | T.T. Dai, L. Liu, D.L. Tao, et al., Influence of Gd doping on the absolute quantumefficiency and lifetime of EuxGd1-x(TTA)3phens, Chin. Chem. Lett. 25 (2014)892-896. |
| [16] | L.Z. Chen, J.J. Mou, Y.Y. Xu, H. Fang, W.F. Xu, Design, synthesis and activity study ofaminopeptidase N targeted 3-amino-2-hydroxy-4-phenyl-butanoic acid derivatives,Drug Discov. Ther. 5 (2011) 61-65. |
| [17] | Y. Ma, Q.Y. Tang, J. Zhu, L.H. Wang, C. Yao, Fluorescent and thermal properties ofsiloxane-polyurethanes based on 1, 8-naphthalimide, Chin. Chem. Lett. 25 (2014)680-686. |
| [18] | M.B. Harbut, G. Velmourougane, G. Reiss, R. Chandramohanadas, D.C. Greenbaum,Development of bestatin-based activity-based probes for metallo-aminopeptidases,Bioorg. Med. Chem. Lett. 18 (2008) 5932-5936. |
| [19] | A. von Wallbrunn, J. Waldeck, C. Hö ltke, et al., In vivo optical imaging of CD13/APN-expression in tumor xenografts, J. Biomed. Opt. 13 (2008) 011007. |
| [20] | Z. Zhang, H. Harada, K. Tanabe, et al., Aminopeptidase N/CD13 targeting fluorescentprobes: synthesis and application to tumor cell imaging, Peptides 26 (2005)2182-2187. |
| [21] | W. Zhang, Z. Ma, L. Du, M. Li, Design strategy for photoinduced electron transferbasedsmall-molecule fluorescent probes of biomacromolecules, Analyst 139(2014) 2641-2649. |
| [22] | L. Chen, W. Sun, W. Li, et al., The first ratiometric fluorescent probe for aminopeptidaseN, Anal. Methods 4 (2012) 2661-2663. |
| [23] | A. Hahnenkamp, M. Schafers, C. Bremer, C. Holtke, Design and synthesis of smallmoleculefluorescent photoprobes targeted to aminopeptdase N (APN/CD13) foroptical imaging of angiogenesis, Bioconjug. Chem. 24 (2013) 1027-1038. |
| [24] | L.Z. Chen, L.P. Du, M.Y. Li, The first inhibitor-based fluorescent imaging probe foraminopeptidase N, Drug Discov. Ther. 7 (2013) 124-125. |
| [25] | H. Umezawa, T. Aoyagi, H. Suda, M. Hamada, T. Takeuchi, Bestatin, an inhibitor ofaminopeptidase B, produced by actinomycetes, J. Antibiot. 29 (1976) 97-99. |
| [26] | W. Xu, Q. Li, Progress in the development of aminopeptidase N (APN/CD13)inhibitors, Curr. Med. Chem. Anticancer Agents 5 (2005) 281-301. |




