b Shanghai Collaborative Innovation Center for Biomanufacturing Technology, Shanghai 200237, China
Since the discovery of imidacloprid [1],the neonicotinoids have been widely used for crop protection and veterinary pest control [2, 3, 4]. However,they are now facing a big challenge arising from growing resistance [5, 6, 7] and severe bee toxicity [8, 9, 10]. On that account,agrochemists have made persistent efforts for the development of novel neonicotinoids which could be more effective and environmental-friendly. In recent years,Li et al. reported pyrrole-fused neonicotinoids [11],hydropyridine-fused neonicotinoids [12, 13, 14] and oxabridged neonicotinoids [15, 16],in all of which the nitro group was located in the same direction as the aromatic heterocycle (cis) [17],and achieved good to excellent insecticidal activity.
Thiazole was a common structural motif in a large number of insecticides,for example,hexythiazox [18],nemathorin [19]. The hotspot on thiazole can be partly ascribed to the capacity in promoting bioactivity,widening insecticidal spectrum and reducing mammal toxicity. Especially in neonicotinoids,2-chloro-5-thiazole ring not only acted as an important role in binding to target,but also benefit to improve selectivity [20, 21, 22, 23]. On this basis,a series of pyrrole-fused new neonicotinoids containing chlorothiazole ring were synthesized,expecting to get excellent results in insecticidal activity and spectrum. 2. Experimental 2.1. Chemistry
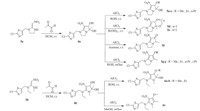
The general synthetic methods for compounds 5a-j and 6a-c are shown in Scheme 1. Unless otherwise noted,reagents and solvents used were from commercial source.
| Download: |
| Scheme 1. Synthetic routes for pyrrole- and dihydropyrrole- fused neonicotinoid analogs. | |
General synthetic procedure for 4a: Starting from 2-chloro-5-chloromethyl-thiazole 1,intermediates 2,3 were obtained in good yields according to reported procedures [11]. Reactant 3a (2.6 g, 10 mmol) was dissolved in 40 mL dichloromethane,then an aqueous solution of methylglyoxal (2.7 g,12 mmol,32%) was added dropwise. The mixture was stirred at room temperature and the progress of the reaction was monitored by TLC. After 45 min, the precipitate was filtered and washed with acetone to afford the white solid 4a(2.52 g,76%).
General synthetic procedure for 5a-f : Compound 4a(0.664 g, 2 mmol) was dissolved in 30 mL alcohol or ketone,then anhydrous aluminum chloride (0.026 mg,0.2 mmol) was added. After stirred at room temperature for 45 min,most of the solvent was removed before adding 30 mL water and then extracted three times with dichloromethane,20 mL for each. The organic layer was combined, dried with anhydrous sodium sulfate,filtered,and concentrated. The residue was purified by silica gel column chromatography to give the desired products 5a-f .
General synthetic procedure for 5g-j: Compound 4a(0.664 g, 2 mmol) was dissolved in 30 mL alcohol,then anhydrous aluminum chloride (0.026 mg,0.2 mmol) was added under reflux. The process was monitored by TLC. After completion,most of the solvent was removed,then extracted three times with dichloromethane,20 mL for each. The organic layer was combined,dried with anhydrous sodium sulfate,filtered,and concentrated. The residue was purified by silica gel column chromatography to give the desired products 5g-j.
General synthetic procedure for 6a-c: Compound 3b (0.828 g, 3 mmol) was dissolved in 30 mL dichloromethane,then an aqueous solution of methylglyoxal (0.810 g,3.6 mmol,32%) was added dropwise. After stirred at room temperature for 45 min, 30 mL water was added,went on stirring for another 15 min before extracted with dichloromethane for five times,20 mL for each. The organic layer was combined,dried with anhydrous sodium sulfate, filtered,and concentrated to obtain a crude product 4b. Compound 4b (0.696 g,2 mmol) was dissolved in 30 mL alcohol,then anhydrous aluminum chloride (0.026 mg,0.2 mmol) was added. After stirred at room temperature (6a-b) or reflux (6c) for 45 min, most of the solvent was removed before adding 30 mL water and then extracted three times with dichloromethane,20 mL for each. The organic layer was combined,dried with anhydrous sodium sulfate,filtered,and concentrated. The residue was purified by silica gel column chromatography to give the desired products 6a-c.
The structures identification data of target compounds are listed in Supporting information. 2.2. Biological assay
All bioassays were performed on representative test organisms grown in the laboratory. The bioassay was repeated at (25±1)°C according to statistical requirements. All compounds were dissolved in N,N-dimethylformamide (AP,Shanghai Chemical Reagent Co.,Ltd., Shanghai,China) and diluted with distilled water containing Triton X-100 (0.1 mg/L) to obtain a series of concentrations of 500.0,100.0,20.0 mg/L and others for bioassays. The insecticidal activity of the synthetic compounds againstAphis craccivoraand Nilaparvata lugenswas tested according to our previous reported procedure [12, 15, 24]. 3. Results and discussion 3.1. Synthesis
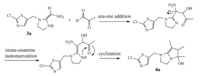
b-Nitro enamine intermediates 3a-b were good electrophilic reagents by reason of highly polarized push-pull ethylene systems. With strong electron-withdrawing group,b-nitro,and electrondonating group,amino group,on different ends,the alkene double bond turned to be polarized owing to electron-transfer delocalization. So that,when methylglyoxal was added as an electrophile with two electrophilic sites of carbonyl,addition reaction came up on both carbonyls,leading to the formation of the cyclized products 4a-b (Scheme 2,illustrated as 4a).
| Download: |
| Scheme 2. Synthetic mechanism of the dihydroxyl dihydropyrrole fused neonicotinoid intermediate. | |
Catalyzed by anhydrous aluminum chloride,dihydroxy-substituted products 4a-b were etherified by different alcohols either at room temperature or under reflux. If etherification occurred at room temperature,dietherified compounds were obtained using 4a as the substrate. However,for 4b,only one of that two hydroxyl groups was successfully etherified to form 6a or 6b in different alcohols under the same condition. Moreover,as 4b were refluxed in methanol,elimination took place at the same time to form aromatized compound 6c. 3.2. Insecticidal activity
Imidacloprid was used as a positive control. The insecticidal activity of the titled compounds againstA. craccivoraandN. lugens was listed in Table 1.
|
Table 1 Insecticidal activities of some compounds against Aphis craccivora. |
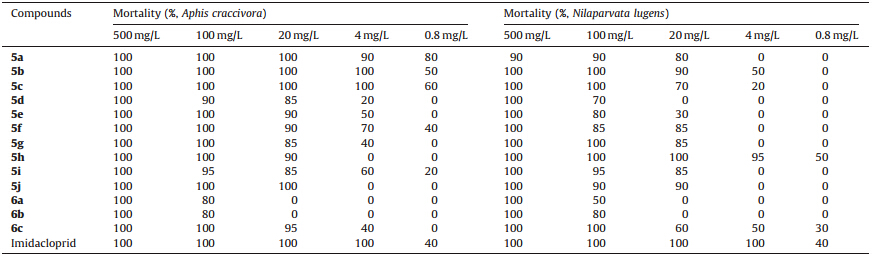
It is delighted to see that most of the compounds showed good insecticidal activity against A. craccivora in Table 1. At a concentration of 0.8 mg/L,some of the compounds,for example 5a-c,still achieved about 60% mortality. Moreover,compounds 5a-jexhibited better bioactivity against A. craccivora than 6a-c, which suggested that cyclic products may achieve higher mortality than acyclic ones. Moreover,compound 5a-c,5h,6c displayed similar insecticidal activity with imidacloprid against N. lugens.
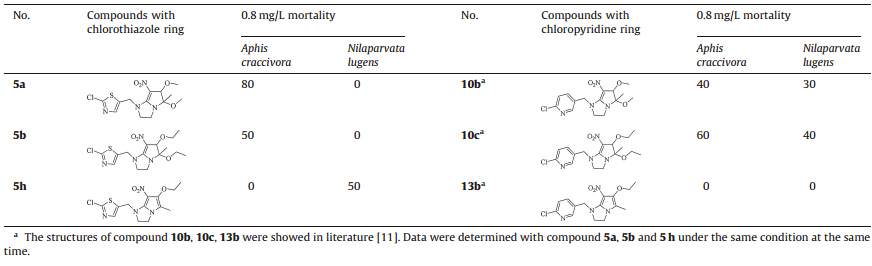
The comparison of compounds with chlorothiazole/chloropyridine ring was listed above. As was shown in Table 2,compound 5a achieved 80% mortality against A. craccivora,better than 10b at a concentration of 0.8 mg/L. For compound 5b,the insecticidal activity against A. craccivorawas almost retained despite the replacement of chloropyridine ring with chlorothiazole. In addition,from the data in Table 2,it showed that the insecticidal activity against N. lugens of compound 5a and 5b decreased obviously compared with the compounds with chloropyridine ring,10b,10c. However,it was gratifying that the mortality of 5c against N. lugens maintained 50%,while that of compound 13b decreased to 0%. It suggested that compound 5h achieved better mortality compared with the analog containing chloropyridine ring. We did not find that the replacement of 2-chloropyridine with chlorothiazole ring brought adverse impact on the insecticidal activity.
|
Table 2 Insecticidal activity comparison of compounds against Aphis craccivoraand Nilaparvata lugens. |
To summarize,a series of pyrrole- and dihydropyrrole-fused neonicotinoid analogs containing chlorothiazole ring were synthesized. The bioassays showed that some of the compounds exhibited excellent insecticidal activity. Compared with imidacloprid,compound 5b and 5c achieved better insecticidal activity against A. craccivora.Compound 5h showed similar insecticidal activity againstN. lugenswith imidacloprid. Compared with the analog containing chloropyridine ring 10b,10c,13b ,compound5a and 5b retained the similar insecticidal activity against A. craccivora.Compound 5h has higher activity than compound 13b against N. lugens. Further researches on the influence of introducing chlorothiazole to bee toxicity are currently in progress.
Acknowledgment
This work was supported by National Key Technology R&D Program of China (No. 2011BAE06B01).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version,at http://dx.doi.org/10.1016/j.cclet.2015. 03.017.
| [1] | S. Kagabu, Discovery of imidacloprid and further developments from strategic molecular designs, J. Agric. Food Chem. 59 (2011) 2887-2896. |
| [2] | I. Ohno,M. Tomizawa, K.A. Durkin, et al., Bis-neonicotinoid insecticides: observed and predicted binding interactions with the nicotinic receptor, Bioorg. Med. Chem. Lett. 19 (2009) 3449-3452. |
| [3] | P. Jeschke, R. Nauen, Neonicotinoids-from zero to hero in insecticide chemistry, Pest Manag. Sci. 64 (2008) 1084-1098. |
| [4] | M. Tomizawa, J.E. Casida, Neonicotinoid insecticide toxicology: mechanisms of selective action, Annu. Rev. Pharmacool. Toxicol. 45 (2005) 247-268. |
| [5] | M. Matsumura, H. Takeuchi, M. Satoh, et al., Species-specific insecticide resistance to imidacloprid and fipronil in the rice planthoppers Nilaparvata lugens and Sogatella furcifera in East and South-east Asia, Pest Manag. Sci. 64 (2008) 1115-1121. |
| [6] | Z.Y. Wang, M.D. Yao, Y.D. Wu, Cross-resistance, inheritance and biochemical mechanisms of imidacloprid resistance in B-biotype Bemisia tabaci, Pest Manag. Sci. 65 (2009) 1189-1194. |
| [7] | K. Gorman, G. Devine, J. Bennison, et al., Report of resistance to the neonicotinoid insecticide imidacloprid in Trialeurodes vaporariorum (Hemiptera: Aleyrodidae), Pest Manag. Sci. 63 (2007) 555-558. |
| [8] | P. Jeschke, R. Nauen, M. Schindler, et al., Overview of the status and global strategy for neonicotinoids, J. Agric. Food Chem. 59 (2011) 2897-2908. |
| [9] | M. Bomgardner, Bee deaths and seed treatments, Chem. Eng. News 90 (2012) 12. |
| [10] | A. Kamel, Refined methodology for the determination of neonicotinoid pesticides and their metabolites in honey bees and bee products by liquid chromatography- tandem mass spectrometry (LC-MS/MS), J. Agric. Food Chem. 58 (2010) 5926-5931. |
| [11] | Z.J. Ye, L.N. Shi, X.S. Shao, et al., Pyrrole- and dihydropyrrole-fused neonicotinoids: design, synthesis, and insecticidal evaluation, J. Agric. Food Chem. 61 (2013) 312-319. |
| [12] | Z.Z. Tian, X.S. Shao, Z. Li, et al., Synthesis, insecticidal activity, and QSAR of novel nitromethylene neonicotinoids with tetrahydropyridine fixed cis configuration and exo-ring ether modification, J. Agric. Food Chem. 55 (2007) 2288-2292. |
| [13] | X.S. Shao, W.W. Zhang, Y.Q. Peng, et al., cis-Nitromethylene neonicotinoids as new nicotinic family: synthesis, structural diversity, and insecticidal evaluation of hexahydroimidazo[1,2-a]pyridine, Bioorg. Med. Chem. Lett. 18 (2008) 6513-6516. |
| [14] | Y.F. Fan, W.W. Zhang, X.S. Shao, et al., Facile three-component synthesis and insecticidal evaluation of hexahydroimidazo[1,2-a]pyridine derivatives, Chin. Chem. Lett. 26 (2015) 1-5. |
| [15] | X.S. Shao, H. Fu, X.Y. Xu, et al., Divalent and oxabridged neonicotinoids constructed by dialdehydes and nitromethylene analogues of imidacloprid: design, synthesis, crystal structure, and insecticidal activities, J. Agric. Food Chem. 58 (2010) 2696-2702. |
| [16] | R.B. Xu, R. Xia, M. Luo, et al., Design, synthesis, crystal structures, and insecticidal activities of eight-membered azabridge neonicotinoid analogues, J. Agric. Food Chem. 62 (2014) 381-390. |
| [17] | X.S. Shao, P.W. Lee, Z.W. Liu, et al., cis-Configuration: a new tactic/rationale for neonicotinoid molecular design, J. Agric. Food Chem. 59 (2011) 2943-2949. |
| [18] | L. Novák, G. Hornyánszky, J. Rohály, et al., Preparation of novel hexythiazox analogues, Pest. Sci. 49 (1997) 85-89. |
| [19] | H. Takahiro, T. Tadaaki, K. Tooru, et al., Organophosphorus compounds as insecticides, miticides, and nematocides, Japan Patent 60104096, 1985. |
| [20] | Y.M. Cui, Q.Q. Huang, J. Xu, et al., Identification of potent type I MetAP inhibitors by simple bioisosteric replacement. Part 1: Synthesis and preliminary SAR studies of thiazole-4-carboxylic acid thiazol-2-ylamide derivatives, Bioorg. Med. Chem. Lett. 15 (2005) 3732-3736. |
| [21] | Q.M. Wang, H. Li, Y.H. Li, et al., Synthesis and herbicidal activity of 2-cyano-3- (2-chlorothiazol-5-yl)methylaminoacrylates, J. Agric. Food Chem. 52 (2004) 1918-1922. |
| [22] | J.G. Samaritoni, D.A. Demeter, J.M. Gifford, et al., Dihydropiperazine neonicotinoid compounds. Synthesis and insecticidal activity, J. Agric. Food Chem. 51 (2003) 3035-3042. |
| [23] | S. Suchail, D. Guez, L.P. Belzunces, Discrepancy between acute and chronic toxicity induced by imidacloprid and its metabolites in Apis mellifera, Environ. Toxicol. Chem. 20 (2001) 2482-2486. |
| [24] | S.Y. Lu, X.S. Shao, Z. Li, et al., Design, synthesis, and particular biological behaviors of chain-opening nitromethylene neonicotinoids with cis configuration, J. Agric. Food Chem. 60 (2012) 322-330. |