With an increasing demand and use of energy worldwide,using semiconductors to harness solar energy has been identified as a viable solution for generating chemical fuels [1],both to provide the driving force for transforming water into hydrogen,as well as a means to provide the oxidizing power for large industrial processes,thereby replacing harsh chemical oxidants [2, 3]. Many metal oxide semiconductors have been interrogated for their chemical stability,optical properties,and surface catalytic reactivity. For example,TiO2 (Eg = 3.0 eV) [4],Fe2O3 (2.2 eV) [5], WO3 (2.7 eV) [6],BiVO4 (2.4 eV) [7],and CuWO4 (2.4 eV) [8] are n-type semiconductors that have been studied for oxidative catalysis such as water oxidation. A large emphasis has been placed on using materials with a band gap between 2 eV and 3 eV since this energy encompasses maximum of the solar spectrum (400-800 nm light comprises ~50% of the solar flux that reaches the earth’s surface) and accounts for reaction overpotential. For these purposes,we bEgan exploring the optical and electronic properties of α-SnWO4 as a thin film electrode.
Despite its calculated band gap of 2.5 eV [9],there are several synthetic challenges for producing α-SnWO4 due to the relative instability of Sn2+ compared to Sn4+. Several groups have produced powder samples of orthorhombic α-SnWO4 as well as cubic β-SnWO4 by calcining in either an inert or vacuum atmosphere as well as by hydrothermal processes [10, 11, 12]. Inert or vacuum atmosphere is necessary because Sn2+ is readily oxidized to Sn4+ in air. There are examples of forming α-SnWO4 by hydrothermal synthesis methods using surfactants under milder,less oxidizing conditions [12, 13]. However,these syntheses start from an amorphous precipitate having a 1:1 Sn:W ratio. Therefore synthetic methods with these restrictions become increasingly more complicated when extrapolated to thin film synthesis. The most common technique to produce thin film electrodes is depositing a solution of dissolved precursors onto a conducting substrate,followed by high temperature calcination and annealing. In the case of generating SnWO4,the precipitation reaction to generate powder takes place if both precursors are co-dissolved (SnCl2(aq) + Na2WO4→SnWO4(s) + 2NaCl(aq)). Also,solution methods employing WCl6 and SnCl2 carried out in organic solvents (i.e. ethanol) to control morphology are problematic since combustion of the solvent cannot occur in a reducing or vacuum atmosphere. Herein we demonstrate the first synthetic method to produce phase pure α-SnWO4 film electrodes (i.e.—not produced from a powder slurry) by a simple hydrothermal method on a fluorinated tin oxide (FTO) transparent conducting substrate without using inert or vacuum atmosphere. This synthesis was accomplished by first synthesizing monoclinic WO3 on FTO and converting it to α-SnWO4 in an aqueous SnCl2 solution. 2. Experimental
All reagents were purchased from Sigma Aldrich and used as received with no further purification. FTO films (Pilkington glass) were washed with ethanol and acetone and sonicated for 15 min each. WO3 was prepared by a known procedure [14]. Sodium tungstate dihydrate,Na2WO4·2H2O,(0.308 g,9.34×102 mmol) was dissolved in 40 mL deionized water. 3 mol/L HCl (13.34 mL, 4×104 mmol) was added dropwise to the stirring solution,which formed a yellow precipitate. Next,ammonium oxalate, (NH4)2C2O4,(0.267 g,2.15×103 mmol) was added and the solution became clear and colorless. An additional 40 mL of deionized water was added,and the solution was stirred for 30 min. Then,11.5 mL aliquots of solution were transferred to 23 mL PTFE liners (Parr Instrument Company). The FTO films were masked off with PTFE tape to a surface area of 1 cm2 so that deposition onto the FTO area was controlled. The films were placed face down in the liner and sealed in a stainless steel autoclave. The vessels were held at 120 ℃ for 12 h with a 10 ℃/min ramp rate. These films were either kept as is (WO3·H2O,yellow/green in appearance) or annealed in air at 450 ℃ for 1 h (monoclinic WO3, green/white in appearance). A 0.5 mol/L SnCl2 solution was made that had an initial pH of ~1. Next the WO3·H2O or WO3 films were placed face down in the hydrothermal vessel filled with 14 mL of 0.5 mol/L SnCl2 solution whose pH was 1 or adjusted to 4 or 7 with 7 mol/L NaOH and 3 mol/L HCl. The vessels were sealed in steal autoclaves and heated for 24 h at 180 ℃ with a 10 ℃/min ramp rate. The films were rinsed with 3 mol/L HCl to remove any tin chloride hydroxide crystals that formed during the hydrothermal reaction (Fig. S1 in Supporting information). In a control experimentWO3 films were placed face down in the hydrothermal vessel filled with 14 mL of water adjusted to pH 7 with 7 mol/L NaOH. This control experiment was also carried out in 1 mol/L NaCl to keep the ionic strength of chloride constant in the reaction.
X-ray diffraction patterns were recorded on a Bruker D8 Advance diffractometer equipped with a graphite monochromator, a Lynx-Eye detector,and parallel beam optics using Cu-Ka radiation (λ = 1.54184Å ). Patterns were collected using a 0.6 mm incidence slit,with a step size and scan rate of 0.04°/step and 0.5 s/step,respectively. Phases were identified as WO3·H2O (JCPDF 43-0679),WO3 (JCPDF 72-1465),and SnWO4 (JCPDF 70-1049) using MDI Jade version 5.0. UV-vis spectra were recorded using a Cary 5000 spectrophotometer (Agilent) equipped with an external diffuse reflectance accessory. Spectra were recorded in reflectance mode,and Tauc plots were then generated using the Kubelka- Munk function,F(R) = (1-R)2/2R. Scanning electron microscope images were collected using a FEI Nova Nanolab SEM/FIB with an accelerating voltage of 15 kV.
Photoelectrochemical (PEC) measurements were performed using a custom-built,three-electrode Pyrex glass cell with a quartz viewing window. The cell contained the working thin-film SnWO4 photoelectrode,an Ag/AgCl reference electrode in saturated KCl (+0.20 V vs. NHE),and a platinum auxiliary electrode. For water oxidation reactions,the supporting electrolyte used was a 0.1 mol/L potassium phosphate buffer (KPi) solution at pH 5.00. Electrical contact was made to the FTO substrate by attaching copper wire purchased from Fisher Scientific and using CG Electronics Silver Print II. Electrodes were sealed using Loctite Hysol 1C epoxy. The light source was a Newport-Oriel 150W Xe arc lamp fitted with a Newport AM 1.5 G filter to simulate incident solar radiation. The lamppowerwas adjusted to 100 mW/cm2 using a Newport 1918-R optical power meter equipped with a Newport 818P-015-19 thermopile detector. Voltammetry measurements were performed with a CH Instruments 660C Electrochemical Workstation. 3. Results and discussion
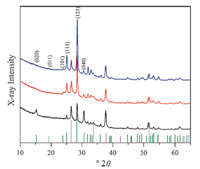
The goal of this work is to synthesize α-SnWO4 electrodes using a simple method that excludes the need for an inert or vacuum atmosphere,and avoids adding an annealing step. We synthesized pure α-SnWO4 films in aqueous SnCl2 solution from a previously synthesized monoclinic WO3 film. The solution did not require deaeration or handling under inert conditions,as all of the synthetic steps were performed aerobically. Orthorhombic WO3·H2O electrodes were generated by a known hydrothermal synthesis method. This compound is composed of layers of corner sharing octahedrally coordinated WO5(H2O) units [14]. Corner sharingWO6 octahedra comprise the structure after annealing,and a hydrothermal method was employed to convert monoclinicWO3 to red-brown films of α-SnWO4 in a subsequent hydrothermal reaction using a 0.5 mol/L solution of SnCl2 at pH 1,4 and 7. XRD patterns show the complete conversion to α-SnWO4 at each pH (Fig. 1). The hkl reflections are shown for low 2θ values for the purposes of discussion. The final α-SnWO4 product is orthorhombic, but also contains corner-sharingWO6 octahedra with Sn atoms layered in between,illustrated in Scheme 1. The XRD at pH 1 (black trace Fig. 1) displays a difference in peak intensity for low 2θ values compared to pH 4 and 7. Specifically the (0 2 0),(1 1 1),(1 2 1),and (0 4 0) reflections show a change in intensity. Therefore,the preferred orientation or morphology of the film is directed by the pH of the SnCl2 solution. Converting tungsten oxide hydrate (WO3·H2O) films to α-SnWO4 also occurs under similar conditions at pH 1 (Fig. S2 in Supporting information),3,4 and 5 (Fig. S3 in Supporting information). The WO3·H2O films converted to α-SnWO4 at pH 3,4,and 5 exhibit similar intensities to that of the crystalline WO3 conversion to α-SnWO4 at pH 4 and 7. However,WO3·H2O films converted to α-SnWO4 at pH 1 display similar changes in intensity of hkl reflections as seen in the black trace in Fig. 1. The intensities in Fig. S2 are stronger than that of the pH 1 solution for crystalline WO3,which is likely due to the fact that the orthorhombic structure is maintained from WO3·H2O to α-SnWO4 as similar reflections appear in the XRD of the WO3·H2O film before conversion (Fig. S4 in Supporting information).
|
Download:
|
| Fig. 1. X-ray diffraction patterns of pure α-SnWO4 films. From the bottom to the top the pH of the hydrothermal solution was pH 1 (black),pH 4 (red),and pH 7 (blue). FTO peaks are represented by the violet vertical lines and a-SnWO4 is represented by the green vertical lines. | |

|
Download:
|
| Scheme. 1. Packing diagrams of (a) monoclinic WO3; (b) WO3·H2O; (c) α-SnWO4. | |
Both WO3·H2O and α-SnWO4 crystallize as orthorhombic structures,and Sn2+ has been shown to undergo ion-exchange reactions into other hydrated oxide crystal structures [15]. The changes in intensities for eitherWO3·H2O or monoclinicWO3 to a- SnWO4 at pH 1 conversion suggest there is preferred orientation that is different than at higher pH values explored. However,the data discussed in this paper unless otherwise noted refers to conditions and outcomes of the conversion of monoclinic WO3 to orthorhombic α-SnWO4. The proposed conversion reaction is: Sn2+(aq) +WO3(s) + H2O(l)→SnWO4(s) + 2H+(aq). Evidence in support of this reaction is that the pH of the solution after the reaction is consistently lower: it decreases from pH 7 to ~3; pH 4 decreases to ~2.5; and pH 1 decreases to ~0.65. Furthermore, after hydrothermal treatment,the precipitate remaining in the vessel was collected,and it indexed to Sn6O4(OH)4 and SnO by XRD (Fig. S5 in Supporting information). The formation of these precipitates also contributes to a drop in the pH of the solution after the completed hydrothermal reaction. While performing the synthesis and adjusting the pH,we found it important to over adjust the pH to 4.5,then and bring it back down to pH 4.0 by titrating in 3 mol/L HCl. The solution composition,and ultimately the α-SnWO4 conversion,depends on whether NaOH is used to adjust the pH,or if both NaOH and HCl are used. With no added HCl,the precipitate remaining after the hydrothermal reaction is dark grey,and indexes mostly to SnO with some Sn6O4(OH)4 present (Fig. S6 in Supporting information). However,with added HCl adjustment,the precipitate is a peach color and indexes mostly to Sn6O4(OH)4 with some SnO present (Fig. S5),in which case we observe consistent conversion to α-SnWO4.
To understand the mechanism by which Sn6O4(OH)4 and SnO byproducts are formed,two identical 0.5 mol/L SnCl2 solutions were prepared in beakers under ambient room conditions without adding WO3-coated FTO films. The pH of the solution in the first beaker (A) was adjusted to 7 with NaOH only,and the pH in the second beaker (B) was adjusted to 7 using NaOH and HCl. A peachcolored precipitate formed immediately from both solutions. The resulting mixtures were then stirred under ambient conditions for 24 h. The precipitate in beaker A slowly turned light grey and got progressively darker over the course of the 24 h. The dark grey precipitate indexed to SnO,with only a small amount of Sn6O4(OH)4 present. The mixture in beaker B appeared virtually unchanged after stirring for 24 h andwas indexed as Sn6O4(OH)4.We surmise that an equilibrium between Sn6O4(OH)4 and SnO exists according to the equation: Sn6O4(OH)4(s) fi 6SnO(s) + 2H2O(l),and that without added acid,the equilibrium shifts toward SnO formation due to the acidity of the proton in Sn6O4(OH)4(s). However,when HCl is used,the equilibrium is shifted back toward Sn6O4(OH)4. Finally,when NaOH is used alone with Sn2+(aq) in the hydrothermal reaction with WO3,the conversion is inconsistent and contains patches of unconverted material. This unconverted material appears blue,which indicates reduced tungsten to W5+,commonly observed in the tungsten bronzes [16]. It is clear that once SnO is formed,the composition of the solution is no longer effective for transforming WO3 to pure α-SnWO4.
One possible film impurity,besides that of incomplete WO3 conversion,is SnO2 formation due to Sn2+ oxidation under aerobic conditions. Since it would be difficult to identify this phase on the underlying FTO substrate,material comprising the films synthesized at pH 7 was scraped off and characterized by XRD to show that no impurity is obtained (Fig. S7 in Supporting information). Oxidizing Sn2+ is avoided by the use of this hydrothermal method. However,if the hydrothermally synthesized α-SnWO4 product is annealed in air,the material dEgrades and SnO2 is the only identifiable product (Figs. S8 and S9 in Supporting information).
SEM imaging was employed to determine the morphology as a function of pH in the hydrothermal reaction with SnCl2 of which the film XRDs are plotted in Fig. 1. The SEM image of the WO3 film before conversion is shown in Fig. 2a (also representative of the WO3·H2O morphology after the initial hydrothermal method). The morphology at pH 1 shows retention of the platelet morphology, except that it has become spongier in appearance. This change likely arises from an expansion of the crystal structure needed to accommodate Sn2+ and form the orthorhombic structure. The retention of morphology is also corroborated by the retention of the low two theta reflections at (0 2 0) and increase in intensity at (0 4 0) with subsequent decrease in the (1 2 1) and (1 1 1) peaks. There are also belts of material lying across the surface (Fig. 2b), which possibly contribute to the decrease in intensity of the reflections at (0 2 0) that we would expect to appear based upon retaining the orthorhombic structure with preferred orientation in the nanoplatelet morphology.We propose the nanobelts are due to some tungsten oxide dissolution and recrystallization of α-SnWO4 at the surface. A close up of the nanoplatelets before-and-after SnCl2-treatment at pH 1 can be seen in Fig. S10 in Supporting information. Two possibilities for α-SnWO4 formation are (1) dissolution of WO3 and complete recrystallization or (2) formation of α-SnWO4 from Sn2+ diffusion into pitted WO3 nanoplatelets. WO3 is known to undergo an acid-base reaction to yield soluble WO42- in aqueous solution having pH≧5. Therefore as the pH increases,soluble WO42- provides a plausible pathway for α-SnWO4 crystallization. The morphology at pH 4 is more like that of a branched nanowire morphology,and is shown in Fig. 2c. The loss in nanoplatelet morphology is also evident in the XRD for the films at pH 4 shown in the red trace in Fig. 1 as the hkl reflections of (0 2 0) and (0 4 0) show a decrease in intensity as the preferred orientation changes. At pH 7,the film forms dense nanorods,shown in Fig. 2d. In this case the XRD also displays something similar to that of pH 4 as a nanorod morphology is obtained and the hkl reflections of (0 2 0) and (0 4 0) again decrease with increasing (1 1 1) and (1 2 1) peaks. If WO3 films are included at pH 7 without SnCl2 at 180 ℃ for 12,18,and 24 h,the WO3 is no longer present on the FTO after the hydrothermal reaction. The same is true if the reaction is carried out in a solution of 1 mol/L NaCl (same chloride concentration). It is evident that crystal structure of the starting material (monoclinic WO3) must play an important role in the conversion to pure orthorhombic α-SnWO4. Therefore,crystal structure along with solution pH directs morphology and preferred orientation of α-SnWO4 formation. From a synthetic materials standpoint,this experimental evidence supports precedence for conversion of other metal tungstates (i.e.—conversion of monoclinic WO3 to monoclinic NiWO4).

|
Download:
|
| Fig. 2. SEM images of (a) WO3 prepared by annealing at 450 ℃; (b) α-SnWO4 produced at pH 1; (c) pH 4; and (d) pH 7. The scale bar in all images is 2 μm. | |
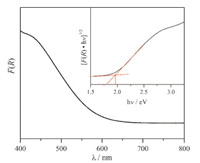
α-SnWO4 has previously been investigated in powder form as a photocatalyst for oxygen evolution from water,and therefore thin films were of interest to us to employ in a photoelectrochemical cell to carry out the reaction. To determine its suitability for the reaction,we first measured the diffuse reflectance UV-vis spectrum,which is plotted in Fig. 3. Notably,the absorption onset is at~650 nm,and α-SnWO4 is determined to be an indirect band gap material with a band-gap energy,Eg = 1.9 eV,shown in the Tauc plot inset of Fig. 3. A 1.9 eV band gap is significantly smaller than many other n-type metal oxide semiconductors such as WO3, BiVO4,and CuWO4,which makes it of interest for further exploration under visible light irradiation conditions.
|
Download:
|
| Fig. 3. Diffuse reflectance UV-vis spectrum (black line). Inset: indirect band-gap Tauc plot. | |
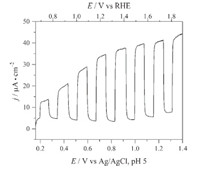
Next,the photoelectrochemical characteristics were measured to test activity for water oxidation. A chopped light linear sweep voltammogram (LSV) at 100 mW/cm2 and AM 1.5G (1-sun) illumination in a 0.1 mol/L KPi buffer solution at pH 5 shows a visible-light response that produces 32 μA/cm2 at the thermodynamic potential for water oxidation,1.23 V vs. RHE (Fig. 4). However,long-term stability of the material in a highly oxidizing atmosphere is necessary for sustainable use. Investigating the material during long-term photoelectrolysis in a pH 5 0.1 mol/L KPi buffer at 1-sun illumination and 1.23 V vs. RHE (Fig. 5) shows a steady decrease in the current response (stability) over the course of 6 h. The data in Figs. 4 and 5 represent films produced at pH 1 with the platelet-like morphology retained. LSV traces under the same conditions were performed on films converted at pH 4 and pH 7,and lower photocurrent was obtained (Fig. S11 in Supporting information). Therefore,the sponge-like platelet morphology must play a role in providing increased catalytic activity. The improved photocurrent observed using the films synthesized at pH 1 could also be due to less exposed FTO,which could reduce recombination at the FTO/α-SnWO4 junction. There is also nonzero current under dark conditions that could arise form Sn2+ oxidation with increasing potential. We note that the standard electrode potential for Sn4+ + 2e-→Sn2+ is only + 0.15 V vs. NHE. This oxidation occurs readily in aqueous conditions,either in the dark or under illumination. However,no oxidation is observed in non-aqueous and air free conditions when performing a cyclic voltammogram (see Supporting information and Fig. S12). It is unknown whether an oxide surface layer forms or if complete oxidation occurs under long-term photoelectrolysis conditions. Future work aims to deposition of a thin oxide material such as TiO2 on the surface of α-SnWO4 to passivate the surface.
|
Download:
|
| Fig. 4. Chopped light linear sweep voltammogram of SnWO4 thin films in 0.1 mol/L KPi buffer at pH 5.00 and AM 1.5G irradiation at 100 mW/cm2. | |

|
Download:
|
| Fig. 5. Bulk electrolysis at 1.23 V vs. RHE of SnWO4 thin films in 0.1 mol/L KPi buffer at pH 5.00 and AM 1.5G irradiation at 100 mW/cm2. | |
Wehave synthesized the first example of thin-film electrodes of the low band gap material α-SnWO4 by a conversion reaction from WO3 and aqueous SnCl2 under hydrothermal conditions. The reaction was carried out under aerobic conditions with no annealing step needed. XRD and SEM images show that the morphology is influenced by changing the solution pH. The band gap of α-SnWO4 is 1.9 eV,suitable for water oxidation,and thin films show a photoelectrochemical response. Although the stability of the films is limited,this chemistry used to develop these films is promising where inert or vacuum atmospheres are not feasible for generating active electrodes. In principle,this preparative method could be adapted for preparing other metal tungstates.
AcknowledgmentsThis work was supported by a grant from a National Science Foundation (No. DMR-1253347). We thank the University of Michigan Department of Chemistry for a Research Excellence Fellowship awarded to K.J.P. and for summer undergraduate support awarded to T.C.E.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2015.01. 027.
| [1] | C. Herrero, B. Lassalle-Kaiser, W. Leibl, A.W. Rutherford, A. Aukauloo, Artificial systems related to light driven electron transfer processes in PSII, Coord. Chem. Rev. 252 (2008) 456-468. |
| [2] | Y. Ren, Z. Ma, P.G. Bruce, Ordered mesoporous metal oxides: synthesis and applications, Chem. Soc. Rev. 42 (2012) 4909-4927. |
| [3] | G.Z. Shen, P.-C. Chen, K. Ryu, C.W. Zhou, Devices and chemical sensing applications of metal oxide nanowires, J. Mater. Chem. 19 (2009) 828-839. |
| [4] | A. Fujishima, K. Honda, Electrochemical photolysis of water at a semiconductor electrode, Nature 283 (1972) 37-38. |
| [5] | K. Sivula, R. Zboril, F.L. Formal, et al., Photoelectrochemical water splitting with mesoporous hematite prepared by a solution-based colloidal approach, J. Am. Chem. Soc. 132 (2010) 7436-7444. |
| [6] | P.P. González-Borrero, F. Sato, A.N. Medina, et al., Optical band-gap determination of nanostructured WO3 film, Appl. Phys. Lett. 96 (2010) 061909. |
| [7] | S.P. Berglund, D.W. Flaherty, N.T. Hahn, A.J. Bard, C.B. Mullins, Photoelectrochemical oxidation of water using nanostructured BiVO4 films, J. Phys. Chem. C 115 (2011) 3794-3802. |
| [8] | J.E. Yourey, K.J. Pyper, J.B. Kurtz, B.M. Bartlett, Chemical stability of CuWO4 for photoelectrochemical water oxidation, J. Phys. Chem. C 117 (2013) 8708-8718. |
| [9] | R. Lacomba-Perales, J. Ruiz-Fuertes, D. Errandonea, D. Martínez-García, A. Segura, Optical absorption of divalent metal tungstates: correlation between the bandgap energy and the cation ionic radius, Europhys. Lett. 83 (2008) 37002. |
| [10] | W. Jeitschko, A.W. Sleight, Synthesis, properties and crystal structure ofb-SnWO4, Acta Cryst. B28 (1972) 3174-3178. |
| [11] | I.-S. Cho, C.H. Kwak, D.W. Kim, S. Lee, K.S. Hong, Photophysical, photoelectrochemical, and photocatalytic properties of novel SnWO4 oxide semiconductors with narrow band gaps, J. Phys. Chem. C 113 (2009) 10647-10653. |
| [12] | H. Dong, Z. Li, Z.X. Ding, et al., Nanoplates of a-SnWO4 and SnW3O9 prepared via a facile hydrothermal method and their gas-sensing property, Sens. Actuators, B: Chem. 140 (2009) 623-628. |
| [13] | G.Q. Zhu, W.X. Que, J. Zhang, P. Zhong, Photocatalytic activity of SnWO4 and SnW3O9 nanostructures prepared by a surfactant-assisted hydrothermal process, Mater. Sci. Eng., B 176 (2011) 1445-1448. |
| [14] | J. Yang, W.Z. Li, J. Li, D.B. Sun, Q.Y. Chen, Hydrothermal synthesis and photoelectrochemical properties of vertically aligned tungsten trioxide (hydrate) plate-like arrays fabricated directly on FTO substrates, J. Mater. Chem. 22 (2012) 17744-17752. |
| [15] | S. Uma, J. Singh, V. Thakral, Facile room temperature ion exchange synthesis of Sn2+ incorporated pyrochlore-type oxides and their photocatalytic activities, Inorg. Chem. 48 (2009) 11624-11630. |
| [16] | P.G. Dickens, M.S. Whittingham, The tungsten bronzes and related compounds, Q. Rev. Chem. Soc. 22 (1968) 30-44. |




