b Department of Chemistry, Nanjing University, Nanjing 210093, China;
c Department of Surgery, University of Michigan, Ann Arbor, MI, USA
Accurate monitoring and control of blood glucose play critical roles in the treatment of patients with diabetes mellitus. Benchtop point-of-care devices are the current standard for many hospitals and medical care facilities,and finger prick glucometers are widely used for personal and home glucose measurements. While these devices yield discrete blood glucose values,trends of rapidly increasing/decreasing glucose concentrations often go unobserved when such devices are used. Some commercially available devices provide continuous glucose monitoring of interstitial fluid using implanted electrochemical sensors. However,due to the~10- 15 min lag time between changes in blood glucose concentration and changes in interstitial fluid glucose [1],in practice these devices can only supplement,rather than fully replace,discrete blood measurements.
Intravenous amperometric glucose sensors may provide a better alternative platform for continuous blood glucose measurements, especially within a hospital environment. Indeed,tight glycemic control is a requirement for many patients in intensive care units (ICUs) to achieve targeted treatment and better patient outcomes. The amperometric glucose sensors described in this work are designed to be implanted intravenously in critical care patients through existing IV port access or inserted into a catheter. This continuous measurement would provide medical staff with the ability to see trends in rising and falling blood glucose,allowing them to select better treatment options. Miniaturized electrochemical blood glucose sensors have been previously reported in literature; however,these sensors can quickly lose analytical accuracy due to thrombus formation on their surfaces when placed within the bloodstream of hospitalized patients [2, 3]. Via the formation of thrombus with encapsulated platelets,the local glucose concentration near the surface of the sensor can be reduced by metabolic activity of platelets and other trapped cells, and therefore,the sensor reads a false glucose value compared to the level of glucose within the plasma phase of the blood. The wellknown antithrombotic and anti-inflammatory properties of nitric oxide provide a useful method of potentially enhancing the hemocompatibility of a blood-contacting surface. Incorporating NO releasing donor molecules into a sensor’s outer polymeric coatings allows it to mimic the functions of endothelial cells lining the inner walls of all blood vessels,which endogenously release NO at localized fluxes of (0.5-4.0)×10-10 mol cm-2 min-1 [4]. This approach has previously been shown to reduce clot formation on the surface of intravenous glucose sensors and preserve their in vivo performance [5, 6]. Herein,we extend this earlier work by further optimizing the NO release formulation and the outer glucose restriction layer used to prepare such devices to achieve more optimal linearity and in vivo performance. 2. Experimental
Glucose oxidase (Type VII,From Aspergillus niger),D-(+)- glucose,glutaraldehyde (25%),bovine serum albumin (BSA), bovine serum (sterile-filtered),sodium chloride,potassium chloride,sodium phosphate dibasic heptahydrate,potassium phosphate monobasic dihydrate,iron (III) chloride (FeCl3),37% hydrochloric acid (HCl),L-ascorbic acid,uric acid,Nafion (5 wt% solution in a lower aliphatic alcohols/H2O mix),m-phenylenediamine, resorcinol,and tetrahydrofuran (THF) were purchased from Sigma-Aldrich (St. Louis,MO). An ester-end capped polylactic acid material (PLA,100 DL 7E) was purchased from Evonik (formerly Lakeshore Biomaterials,Birmingham,AL). E2As Elast-Eon thermoplastic polyurethane was a gift from AorTech International,plc (Scoresby,Victoria,Australia). Diazeniumdiolated N,N0-dibutyL-1,6-hexanediamine (DBHD) or DBHD/N2O2 was synthesized by treating DBHD with 80 psi NO gas purchased from cryogenic gases (Detroit,MI) at room temperature for 24 h, as previously described [7]. All amperometric measurements were collected with a 4-channel ESA BioStat potentiostat instrument.
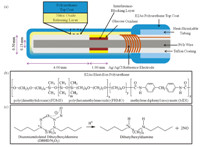
Glucose sensors were constructed based on previous designs [5, 6, 8, 9] by first cutting a 1-mm length cavity in the Teflon coating of the platinum/iridium wire (outer diameter = 0.2 mm). A Nafion coating was applied to the cavity,and then Via a CV electropolymerization process (cycling voltage between 0 and +830 mVat 2mV s-1 for 18 h) a layer of polymerized resorcinol and mphenylenediamine [10, 11] was applied to the cavity to help reject electroactive interference species,such as ascorbic acid,uric acid, and acetaminophen from reaching the Pt/Ir surface. A silver/silver chloride (Ag/AgCl) wire electrode was tightly wrapped around the sensor to serve as an electrochemical reference,and heatshrinkable polyester tubing was applied to secure the reference wire in place (Fig. 1a). Glucose oxidase was then immobilized within the cavity using glutaraldehyde. Outer layers were then applied to the sensor’s surface using a wire loop: first,an estercapped polylactic acid layer containing diazeniumdiolated dibutylhexyldiamine (2:1,wt/wt) (Fig. 1c) was applied to give the sensor NO releasing behavior,and then a 2% (wt/vol) Elast-Eon E2As polyurethane in THF (Fig. 1b) was applied as a top coat to modulate the NO release and also control the sensor’s linear detection range for glucose by restricting glucose diffusion into the enzyme layer. E2As polyurethane was previously used with positive success as an NO release coating to prevent platelet activation and clotting within a rabbit model for extracorporeal circulation [12]. A diagram of the fully-assembled sensor is shown in Fig. 1a. The control sensors were prepared by similar procedure except that no diazeniumdiolated dibutylhexyldiamine was doped within the PLA coating.
|
Download:
|
| Fig. 1. (a) Needle/catheter type glucose sensor design; (b) E2As Elast-Eon polyurethane used for the sensor outer layer; (c) lipophilic diazeniumdiolated dibutylhexyldiamine and the proton-driven mechanism for nitric oxide release. | |
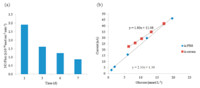
These sensors were soaked in phosphate buffered saline (PBS) or bovine serum at physiological conditions (pH 7.4,37.5 ℃) to hydrate the polyurethane layer for restricted glucose diffusion and to activate the NO release with protons within the PLA layer. The PLA controls the local pH of the NO releasing layer by supplying protons as it slowly hydrolyzes. Ideally performing sensors release NO at a stable flux at physiological levels found for endothelial cells ((0.5-4.0) T 10-10 mol cm-2 min-1) for the duration of the sensor’s functional lifetime. Nitric oxide release from the surface of the sensors was measured by chemiluminescence using a Sievers Nitric Oxide Analyzer (NOA) 300i. The NO release profile over 7 days of the optimized sensors used for the in vivo experiment can be seen in Fig. 2a. The sensorsmaintained NO release above 0.5×10-10 mol cm-2 min-1 over this period while stored in bovine serum solution (containing glucose at physiological levels).Without the addition of PLA,the NO release profile would exhibit an initial burst followed by a much sharper daily decay. As indicated in Fig. 1c,the NO release mechanism from diazeniumdiolated dibutylhexyldiamine is dependent upon a steady supply of protons. In our previously published in vivo glucose sensor experiments,a 50:50,acid-capped poly(lactic-coglycolic acid) (PLGA)was used to formulate theNOrelease layer to control local pH at the sensor surface. The innate acid-terminated polymer chains of this material coupled with the co-polymer structure yield relatively rapid hydrolysis rates; thus,the NO release profile based on PLGA begins with a large burst of NO on day 1 with a characteristic decay over subsequent days. In contrast,the PLA used in the studies reported herein has estercapped end groups,which slow the hydrolysis rate,and therefore provides a more stable NO release profile for the duration of testing. This ensures that NO release will remain greater than 0.5 T 10-10 mol cm-2 min-1 until after the 7th day of sensor lifetime.
|
Download:
|
| Fig. 2. (a) Nitric oxide release fluxes of sensors stored in bovine serum at 37.5 ℃ for three days prior to day 1 testing,and day 7 measurements were conducted after in vivo implantation; (b) calibration curves of representative sensor in both PBS and bovine serum at 37.5 ℃ before in vivo implantation. | |
E2As Elast-Eon,a polymer composed of a mixed soft segment of poly(dimethylsiloxane) and poly(hexamethylene oxide) with a methylene diphenyl isocyanate (MDI) hard segment,was used as the outermost coatings in the fabrication of the glucose sensors because it has been reported to exhibit excellent intrinsic biocompatibility and biostability,with low levels of blood protein adhesion [13, 14]. Since inherent hemocompatibility of the top coat should enhance the overall thromboresistant properties from the NO release devices,E2As was selected as a replacement for PurSil Thermoplastic Silicone Polyether used in our previous in vivo glucose sensor designs [5]. In benchtop studies,the E2As coated sensors displayed the desired control over the linear glucose detection range (slowing glucose diffusion to enzyme layer so sensor is not limited by oxygen levels); the NO release through this layer was also quite acceptable for this in vivo study. The linear range for amperometric glucose response of the NO release sensors was also measured in two types of solutions (PBS and bovine serum) in vitro before and after the in vivo implantation to evaluate their performance and recovery. Ideally,a sensor should have linear response not only within the standard resting blood glucose range of 4.4-6.6 mmol L-1,but it should also provide accurate measurements within the hypoglycemic and hyperglycemic ranges (1.0-20.0 mmol L-1). The sensor should also be selective over other electroactive species present in blood that interfere with the electrochemical oxidation of hydrogen peroxide at the sensor’s operating potential of +600 mV (vs. Ag/AgCl). The glucose calibration curves for a representative sensor tested in vitro within both PBS and bovine serum (Fig. 2b) demonstrate that the sensor has a similar linear range in both environments,although a higher calibration intercept and decreased sensitivity are observed in serum,which may result from the presence of additional unknown interfering species within that commercial serum matrix. Response times for sensors intended for continuous monitoring should also be as short as possible so that they quickly react to rapid blood glucose concentration changes. Accordingly,the typical response times for the sensors employed in the in vitro serum calibration experiment shown in Fig. 2b were 5 min (with response time defined as the time required for the signal to achieve 95% of its final steady-state amperometric value after change in glucose concentration).
When implanted into rabbit jugular veins,the glucose sensors were used to measure the in vivo blood glucose level and generate an amperometric time trace (Fig. 3a). When a bolus of 50% dextrose solution was given intravenously to modulate the rabbit’s venous glucose concentration,the NO releasing sensor responded to the resulting change in blood glucose with a proportional increase in anodic current. Also,as observed in Fig. 3b,after the 7 h in vivo experiment the NO releasing sensor does not develop significant clotting on its surface; hence,the sensor’s surface will be in direct contact with the blood,and the observed current will remain proportional to the true blood glucose value. In contrast,the control sensor without NO release quickly developed a surface clot, as shown in Fig. 3b. The contrast between the clotting patterns of the control and NO release sensors is very similar to results obtained from previous glucose sensors that were evaluated in vivo Via rabbit studies [5]. Therefore,this behavior is representative of the expected clotting that will be observed when additional sensors with the optimized outer layers are tested with many future in vivo animal studies. The cells within the clot (e.g., platelets) consume glucose in the localized sensing area and this also creates an additional diffusion barrier,resulting in very low currents for the control sensor when placed in vivo. It should be noted that due to anesthesia,the rabbit’s glucose levels start at hyperglycemic levels (13.8 mmol L-1),and then drop with time until dextrose is infused.

|
Download:
|
| Fig. 3. (a) Raw current time trace of in vivo implanted sensors. Two injections of 50% dextrose were given to modulate the blood glucose of the rabbit; (b) photos of the control (top) and NO release (bottom) glucose sensors after the in vivo experiment. The portions of the sensors to the left of the dashed lines were actually inside the veins. | |
To assess the glucose calibration accuracy,the discrete venous blood glucose values,taken by an ALB-800 Flex Radiometer blood gas analyzer,were compared with the continuous measurements provided by the sensors. Both a one-point calibration based upon the venous blood glucose at the 1 h time-point and the in vitro calibration of the NO releasing sensor in bovine serum were used to convert the raw current values to corresponding glucose concentrations. In Fig. 4,the discrete venous blood glucose values are compared with the continuous glucose measurements, converted by a one-point calibration and the in vitro bovine serum calibration for the NO releasing sensor and by a one-point calibration for the control sensor. The NO releasing sensor quickly responds when a bolus of 50% dextrose is given intravenously,and the calibrated glucose values more closely match those of the blood gas analyzer than those of the control sensor. The delay in response time to the dextrose boluses and the deViations from the venous blood glucose concentration by the control sensor are likely due to the heavy surface clot formation (Fig. 3b).

|
Download:
|
| Fig. 4. Comparison of glucose concentration values obtained from benchtop blood gas analyzer and the converted current values measured by the continuous sensor. One conversion of current to glucose concentration (mmol L-1) was made with the calibration curve in bovine serum (Fig. 2); the other conversion was a one point calibration taken at the 1 h time point. | |
Thus,nitric oxide release provides the sensor with resistance to in vivo thrombosis,allowing the sensor to maintain continuous analytical functionality and measurement accuracy for the duration of its implantation. The one-point blood gas calibration and the bovine serum calibration both yielded similar values when used to convert the raw current values obtained from the NOreleasing sensor. 4. Conclusion
In summary,the novel incorporation of E2As Elast-Eon as an outer coating and ester-capped PLA as a layer containing the NO release diazeniumdiolate species appears to provide an ideal combination coating to create improved intravascular electrochemical glucose sensors. The use of E2As material serves a critical role by maintaining linear response to venous blood glucose concentrations up to 20 mmol L-1 through its restriction of glucose diffusion to the glucose oxidase layer. The PLA serves to maintain a local acidic environment within the layer containing diazeniumdiolated DBHD,so that NO release is prolonged. The resulting NO release profile is sufficient to allow the continuous monitoring electrochemical glucose sensors to mimic the behavior of endothelial cells,granting them resistance to surface clot formation. This thromboresistance is integral to preserve in vivo analytical performance and functionality. The accuracy of NO-releasing glucose sensors was assessed by implantation within rabbit veins. Modulation of the animal’s venous blood glucose with dextrose bolus infusions can be used to help determine glucose sensor response time. Control sensors quickly developed surface clots that increase response time and decrease their measurement accuracy. NO release functionality helps prevent surface clot formation which preserves fast sensor response time and glucose concentration measurement accuracy when results are compared with whole blood analyzer values obtained with discrete blood samples drawn from the animal. In this preliminary study,optimizing the NO release formulation and outer glucose restriction layer used to fabricate these devices has yielded improved in vivo performance. For future in vivo experiments,it is expected that both the thromboresistance and improved analytical measurement accuracy will be observed with additional sensors utilizing this novel design.
AcknowledgmentWe thank the Leona M. & Harry S. Helmsley Charitable Trust for support of this work.
| [1] | M.S. Boyne, D.M. Silver, J. Kaplan, C.D. Saudek, Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor, Diabetes 52 (2003) 2790-2794. |
| [2] | N. Wisniewski, M. Reichert, Methods for reducing biosensor membrane biofouling, Colloid Surf. B: Biointerfaces 18 (2000) 197-219. |
| [3] | M. Frost, M.E. Meyerhoff, In vivo chemical sensors: tackling biocompatibility, Anal. Chem. 78 (2006) 7370-7377. |
| [4] | M.W. Vaughn, L. Kuo, J.C. Liao, Estimation of nitric oxide production and reaction rates in tissue by use of a mathematical model, Am. J. Physiol. 274 (1998) H2163-H2176. |
| [5] | Q. Yan, T.C. Major, R.H. Bartlett, M.E. Meyerhoff, Intravascular glucose/lactate sensors prepared with nitric oxide releasing poly (lactide-co-glycolide)-based coatings for enhanced biocompatibility, Biosens. Bioelectron. 26 (2011) 4276-4282. |
| [6] | Q. Yan, B. Peng, S. Gang, et al., Measurement of tear glucose levels with amperometric glucose biosensor/capillary tube configuration, Anal. Chem. 83 (2011) 8341-8346. |
| [7] | M.M. Batchelor, S.L. Reoma, P.S. Fleser, et al., More lipophilic dialkyldiaminebased diazeniumdiolates: synthesis, characterization, and application in preparing thromboresistant nitric oxide release polymeric coatings, J. Med. Chem. 46 (2003) 5153-5161. |
| [8] | D.S. Bindra, Y.N. Zhang, G.S. Wilson, et al., Design and in vitro studies of a needle-type glucose sensor for subcutaneous monitoring, Anal. Chem. 63 (1991) 1692-1696. |
| [9] | R. Gifford, M.M. Batchelor, Y. Lee, et al., Mediation of in vivo glucose sensor inflammatory response via nitric oxide release, J. Biomed. Mater. Res. A 75A (2005) 755-766. |
| [10] | I. Carelli, I. Chiarotto, I. Curulli, G. Palleschi, Electropolymerization of hydroxybenzene and aminobenzene isomers on platinum electrodes to assemble interference- free electrochemical biosensors, Electrochim. Acta 41 (1996) 1793-1800. |
| [11] | R.J. Geise, J.M. Adams, N.J. Barone, A.M. Yacynych, Electropolymerized films to prevent interferences and electrode fouling in biosensors, Biosens. Bioelectron. 6 (1991) 151-160. |
| [12] | E.J. Brisbois, H. Handa, T.C. Major, R.H. Bartlett, M.E. Meyerhoff, Long-term nitric oxide release and elevated temperature stability with S-nitroso-N-acetylpenicillamine (SNAP)-doped elast-eon E2As polymer, Biomaterials 34 (2013) 6957-6966. |
| [13] | D. Cozzens, A. Luk, U. Ojha, M. Ruths, R. Faust, Surface characterization and protein interactions of segmented polyisobutylene-based thermoplastic polyurethanes, Langmuir 27 (2011) 14160-14168. |
| [14] | A. Simmons, A.D. Padsalgikar, L.M. Ferris, L.A. Poole-Warren, Biostability and biological performance of a PDMS-based polyurethane for controlled drug release, Biomaterials 29 (2008) 2987-2995. |




