Metallacrowns (MCs,Fig. 1) are a class of self-assembled coordination macrocycle possessing an inorganic ring that resembles crown ethers [1, 2]. MCs often possess useful physical properties originating from their inorganic ring,such as singlemolecule magnetism [3, 4, 5, 6, 7] and luminescence [8, 9]. Open metal sites on MCs allow for the synthesis of elaborate solid-state architectures from MC building blocks [10, 11, 12]. Selective anion binding and chiral recognition has also been achieved in MC metallocavitands adorned with hydrophobic chiral ligands,such as L-phenylalanine hydroxamic acid (pheHA) (Fig. 1) [13, 14, 15, 16].

|
Download:
|
| Fig. 1. Chemdraw diagram showing the picHA and pheHA fused chelate rings promote the formation of Ln(III)[15-MCZn(II),picHA)-5]3+ complexes. | |
Such diverse MC chemistry has motivated extensive research into controlling their assembly. In particular,the promise of tuning the physical properties of MCs through ring ion substitution has proven useful for magnetism and luminescence applications. We’ve recently prepared lanthanide MCs (LnMCs) with some of the brightest reported near-infrared luminescence by using Zn(II) as the ring ion in place of the more commonly utilized Cu(II) and Ni(II) [8, 9]. The use of the d10 Zn(II) ion avoided the luminescence quenching that typically occurs through d-d relaxation in Cu(II) and Ni(II) 3d-4f complexes [17, 18].
Ring ion substitution can drastically alter metallacrown assembly. The only LnMC obtained from the reaction of picHA, Cu(II) or Ni(II),and a Ln(III) ion is the Ln(III)[15-MCM(II),picHA)-5]3+ shown in Fig. 1 [19, 20, 21, 22]. With Zn(II),eight distinct LnMC motifs have been identified by electrospray ionization mass spectrometry (ESI-MS),including the Ln(III)[15-MCZn(II),picHA)-5]3+ [23]. Five of these complexes have been isolated and crystallographically characterized [8, 24, 25]. Such structural variety compared to Cu(II) and Zn(II) was attributed to the preference for Zn(II) to form nonplanar 5-coordinate geometries with picHA [26],which ensures that no single complex is thermodynamically preferred based on the geometric principles established for coordination driven assembly [27]. In addition,Zn(II) has been used to template inverse metallacrowns [28].
To expand on these luminescence and speciation results,we have examined the assembly of LnMCs with Zn(II) ring ions using pheHA,a structural analog of picHA that possesses a more basic amine in place of the pyridyl group. With Cu(II) and Ni(II),the two ligands yield similar MCs based on ESI-MS,crystallography,and the fitting of potentiometric titrations [19, 29, 30, 31, 32, 33]. We use ESI-MS to demonstrate that the reaction of Zn(II),pheHA,and Ln(III) ions yield two LnMCs and a Zn5L42+ intermediate observed with picHA following a similar solvent dependent relationship. An unprecedented Ln(III)[16-MCZn(II),pheHA,HpheHA-6]5+ was crystallographically characterized. This LnMC assembly behavior highlights the importance of the metal coordination geometry and ligand basicity for controlling ring ion substitutions. 2. Experimental 2.1. Mass spectrometry
Electrospray ionization mass spectrometry (ESI-MS) was performed with a Micromass LCT time of flight electrospray ionization mass spectrometer at 150℃ at cone voltages ranging from-100 V to 100 V. No negatively charged species were evident using negative cone voltages. For the assembly of Zn(II) and L-pheHA,the samples were prepared by mixing L-pheHA [34] (15.0 mg,83.2 μmol),Zn(NO3)2·(H2O)6 (30.9 mg,104 μmol),and triethylamine (23.2 μL,167 μmol) in 5 mL of pyridine at room temperature and stirring overnight. For the assembly of Zn(II), L-pheHA,and Ln(III) ions,the samples were prepared by mixing L-pheHA (15.0 mg,83.2 μmol),Zn(NO3)2·(H2O)6 (24.7 mg, 83.2 μmol),the respective Ln(NO3)3 salt (16.6 μmol),and triethylamine (23.2 μL,167 μmol) in 5 mL of pyridine at room temperature and stirring overnight. The homogeneous solutions were diluted 20-fold and injected into the ESI-MS via syringe pump. ESIMS data was processed with MassLynx 4.0 software. 2.2. LaZn4(pheHA)2(HpheHA)3(NO3)5(pyridine)7
In a 25 μL Ehrleneyer flask,pheHA [34] (200 mg,11.1 μmol), Zn(NO3)2·(H2O)6 (330 mg,11.1 μmol),La(NO3)2·(H2O)6 (95 mg, 0.22 μmol),and triethylamine (312 μL,2.22 μmol) were combined and stirred in 12 μL of pyridine overnight. The homogeneous solution was distributed into three 20 μL glass vials,which were placed in a wide-mouth jar containing 100 μL of diethyl ether. Colorless crystals formed within 24 h. The crystals were highly deliquescent,which prevented the isolation of analytically pure samples. ESI-MS of the solid dissolved in pyridine did not reveal any peaks for the parentLaZn4(pheHA)2(HpheHA)2 complex. 2.3. Crystallography
Intensity data for LaZn4(pheHA)2(HpheHA)2(NO3)5(pyridine)7 was collected at 85 K on a standard Bruker SMART-APEX CCDbased X-ray diffractometer equipped with a low temperature device and fine focus Mo-target X-ray tube (λ = 0.71073Å ) operated at 1500W power (50 kV,30 mA). The frames were integrated with the Bruker SAINT [35] software package with a narrow frame algorithm. The data were processed with SADABS [36] and corrected for absorption. All structures were solved and refined with the Bruker SHELXTL software package [37]. All nonhydrogen atoms were refined anisotropically. Hydrogen atoms were placed in their idealized positions. For a crystal of dimensions 0.22 mm × 0.12 mm × 0.12 mm,the structure was solved with formula LaZn4C91H97N24O23,M = 2295.32 g/mol. Trigonal space group P32,a = 15.5277(2)Å ,b = 15.5277(2)Å ,c = 35.883(3)Å , V = 7492.7(6)Å3,Z = 3,ρcalcd = 1.526 mg/cm3,μ = 5.091 mm-1, max. and min. transmission 0.5853 and 0.4060 respectively,580 restraints,1360 parameters,77,663 reflections (R(int) = 0.0650), 17,767 independent reflections,for observed data R1 = 0.0493, wR2 = 0.1266,for all data R1 = 0.0533,wR2 = 0.1305,largest diff. peak and hole 1.120 and-1.006 eÅ /cm3. 3. Results and discussion
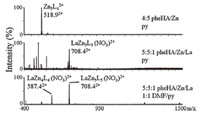
The assembly of MCs with Zn(NO3)2 and pheHA was examined in a variety of solvents using ESI-MS,with samples being injected into the instrument via a syringe pump. We’ve found previously that the speciation observed by ESI-MS is highly solvent dependent,and syringe pump injection provides better control over complex stability and assembly than the more common chromatographic injection methods [25]. MCs are typically positively charged species that possess distinctly high molecular weights and broad isotope distributions,which makes their identification facile by ESI-MS. For the assembly of Zn(NO3)2 and pheHA using triethylamine as a base at room temperature,MC peaks were only obtained in pyridine or mixtures of pyridine and other solvents (Fig. 2). The only MC species present in the samples was a Zn5 (pheHA)42+ ,which presented peaks for the solitary dication,pyridine adducts,or a + 1 charged cation with an associated NO3- anion. No new species were observed by ESIMS upon heating the reaction mixture at 65 ℃. The Zn5 (pheHA)42+ is likely the Zn(II)[12-MCZn(II),pheHA-4]2+ complex based on the observation of this complex with other ligands and Cu(II) and Ni(II) ions [30, 31, 32, 33, 38, 39, 40, 41, 42, 43, 44]. Extensive effort dedicated to crystallizing this complex proved unsuccessful.
|
Download:
|
| Fig. 2. ESI-MS of room temperature assembly reactions in the indicated stoichiometry and solvents. | |
The assembly of 5 equiv. of pheHA,5 equiv. of Zn(NO3)2, 10 equiv. of triethylamine,and 1 equiv. of Ln(NO3)3 was performed in pyridine at room temperature. ESI-MS revealed peaks for a LaZn5(pheHA)5 3+ species,with the highest intensity peaks being +2 charged adducts with associated NO3- or OH- anions (Fig. 2). This speciation is consistent with a Ln(III)[15-MCZn(II),pheHA-5]3+ complex. Other solvents were explored to determine if the solvent dependence observed with picHA [25] occurred with a-aminoHA ligands. In 1:1 DMF/pyridine (v/v),ESI-MS revealed the Zn5 (pheHA)42+ ,LaZn5(pheHA)5 3+ species previously observed in pure pyridine. Additionally,prominent peaks for an LaZn4(- pheHA)4 3+ are observed (Fig. 2). This observation parallels the findings with picHA,in which an Ln(III)[12-MCZn(II),picHA-4]3+ assembles in DMF/pyridine mixtures. No other MC species were evident by ESI-MS in any solvent.
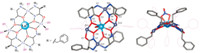
We attempted to crystallize the Ln(III)[15-MCZn(II),pheHA-5]3+, and promising colorless crystals were obtained by ether vapor diffusion. Surprisingly,the crystal structure revealed an unprecedented complex with the formula LaZn4(pheHA)2(HpheHA)2(NO3)5(pyridine)7. Using metallacrown nomenclature,the complex is best described as a La(III)(NO3)[16-MCZn(II)pheHA,HpheHA,- 6](NO3)4,the first [16-MC-6] to be reported (Fig. 3). Unlike the high rotational symmetry of most metallacrowns with hydroxamic acid ligands,this complex has only a C2-axis of rotation. The prototypical [M-O-N] repeating unit in the MC ring that involves the oxime group in the hydroximate ligand is disrupted because two distinct pheHA rotamers are coordinated in the complex. The complex instead possesses an [M-O-N-M-O-C-N-O] repeat unit (Figs. 3 and 4). The overall complex is saddle-shaped,which is promoted by the distorted five-coordinate Zn(II) ring ions (Fig. 5A and B). The phenyl side chains on pheHA have opposite facial orientations around the MC ring,which contrasts the facedifferentiation seen in chiral Ln(III)[15-MCCu(II),α-aminoHA-5]3+ and Cu(II)[15-MCCu(II),β-aminoHA-5]2+ complexes [45, 46]. Five nitrate anions were identified in the structure,which indicates that two of the pheHA ligands are protonated. We are assigning the non-coordinating Noxime atoms on two pheHA ligands as the protonation sites based on the close hydrogen bonding from pyridine molecules in the lattice (NOxime-Npy = 2.84Å ,Fig. 5C). This assignment is supported by findings from other hydroximate complexes [47].
|
Download:
|
| Fig. 3. Chemdraw diagram and crystal structure of the La(III)[16-MCZn(II),pheHA,HpheHA-6](NO3)5(py)7. Select hydrogens were removed for clarity. Color scheme: Light blue = La(III),Gray sphere = Zn(II),red = oxygen,blue = nitrogen,white = hydrogen,gray lines = carbon,thin purple line = pyridine ligands. | |
|
Download:
|
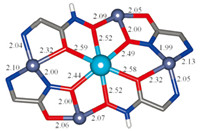
| Fig. 4. Structure of the La(III)[16-MCZn(II),pheHA,HpheHA-6](NO3)5(py)7 core highlighting the metal-ligand bond distances. | |
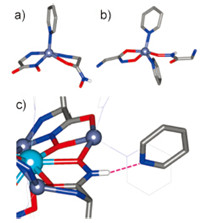
The central La(III) ion is 9-coordinate,with 6 oxygen donor atoms from the MC ring (4 Ooxime,2 Ocarbonyl). The average La- Ooxime bond distances (Fig. 4) with the doubly-deprotonated pheHA ligands are noticeably shorter than the average La-Ooxime bonds with the singly deprotonated HpheHA ligands and the La- Ocarbonyl distances (2.46,2.51,and 2.58Å ,respectively). These differences reflect the-2 charge on pheHA vs.-1 on HpheHA,as well as greater charge delocalization to the Ooxime than the Ocarbonyl atoms. The La(III) ion forms equatorial 4-membered,5-membered, and 6-membered chelate rings with the MC architecture. A bidentate nitrate and pyridine are coordinated axially. La(III) ions typically prefer oxygen donor atoms,which is reflected in the long La-Npy bond distance of 2.716Å . Two distinct Zn(II) coordination environments are evident,both of which are 5-coordinate. One contains a distorted square pyramidal Zn(II) based on the t-value[48] of 0.24,0.33 (Fig. 5A) with an axial pyridine and equatorial bidentate picHA ligands (one Namine,N' oxime chelate,one Namine,Ooxime chelate). The Zn-ligand bond distances are within the expected range for a five-coordinate Zn(II) ion (Fig. 4). The two other Zn(II) ions are coordinated by two equatorial pyridine ligands,a bidentate Ooxime,Ocarbonyl pheHA ligand,and an Ooxime from another pheHA ligand (Fig. 5B). Based on their t-values of 0.47 and 0.59,these ions are intermediate between pure squarepyramidal and trigonal bipyramidal geometry. The Zn-Ocarbonyl bond distance of 2.32Å is exceptionally long,and may be due to both the geometric strain in forcing the pheHA ligand into a [16-MC-6] motif or that the Ocarbonyl also coordinates to the central La(III) ion.
|
Download:
|
| Fig. 5. Coordination geometry of (A) Zn1 and Zn3 (t = 0.59 and 0.47) and (B) Zn2 and Zn4 (t = 0.33 and 0.24) and (C) highlight of the hydrogen bond between a solvent pyridine and the hydroxamate nitrogen. | |
The LaZn4(pheHA)2(HpheHA)25+ complex possessed no appreciable solution stability. No ESI-MS peaks for the complex could be observed upon dissolution of the crystalline material in pyridine. Instead,peaks for the LaZn5(pheHA)5 3+ and Zn5(pheHA)42+ were evident. We suspect its crystallization is kinetically driven. Nevertheless,the complex crystallized persistently. Substituting 4-methylpyridine for pyridine in the reaction mixture yielded an identical LaZn4(pheHA)2(HpheHA)25+ . Also,the complex was obtained with a bidentate carboxylate guest (bis(4-[2-(4-pyidyl) ethenyl]benzoic acid) [49] coordinated to the central La(III) in place of the nitrate anion. The persistence of the LaZn4(phe- HA)2(HpheHA)25+ prevented crystallographic characterization of the LaZn5(pheHA)5 3+ and LaZn4(pheHA)4 3+ complexes. The primary aim of this work is to gain insight into how ring metal substitutions influence MC assembly and structure. Previous work with a-aminoHA ligands have focused on Cu(II),and Ni(II) ring ions,which react with Ln(III) ions to form stable Ln(III)[15- MCM(II),a-aminoHA-5]3+ complexes exclusively and exhibit no solvent dependent assembly behavior [19, 22, 24]. With coordination macrocycles,the geometry of the complex is dependent on the geometry of the metal ion and ligand building blocks [50]. a-AminoHA ligands,such as pheHA,generate a 108° vertex between their fused chelate rings (Fig. 1). This angle is compatible with the internal angle of a pentagon,which means that pheHA thermodynamically favors M(N)[15-MCM'(N'),pheHA-5]N+ complexes (Fig. 1). Therefore,the observation of LaZn5(pheHA)5 3+ complexes by ESI-MS (Fig. 2),consistent with a Ln(III)[15-MCZn(II),pheHA-5]3+ motif,is unsurprising. This complex was also observed using ESIMS and crystallographically characterized with the picHA ligand [24]. However,the preferred coordination geometries of Zn(II) are incompatible with a pentagonal Ln(III)[15-MCZn(II),pheHA-5]3+ based on symmetry considerations. The Zn(II) ring ions possess five-coordinate geometries that result in a non-planar arrangement of the pheHA ligands. With the picHA ligand,the fivecoordinate Zn(II) ions generated concave M(N)[12-MCZn(II),picHA- 4]N+ motifs (Fig. 1). Likely,the Zn5(pheHA)4 2+ and LaZn4(pheHA)43+ complexes observed by ESI-MS possess a similar structure. Notably,the preferential assembly of the LaZn4(pheHA)43+ in DMF/pyridine mixtures was also encountered with picHA, generalizing our previous finding that symmetry incompatible building blocks promote solvent-dependent assembly [25].
The incompatible symmetry of the pheHA and Zn(II) building blocks ensures that every possible metallamacrocycle that assembles will suffer from appreciable geometric strain. With picHA,this strain was presented by long Zn-NpicHA-pyridyl bond distances of ~2.15Å ,compared to ~2.05Å for the bond between Zn(II) and the nitrogen on coordinated pyridine ligands. On pheHA, this pyridyl nitrogen is substituted for a more basic amine ligand (pKa of pyridinium = 5.2,pKa of pheHA ammonium = 9.8 [15]). The pheHA amine group possesses greater metal ion affinity than the corresponding picHA pyridyl group,which concomitantly disfavors the elongation of the Zn-NpheHA-amine bonds.
This difference in ligand basicity can explain why the La(III) [16-MC-Zn(II),pheHA,HpheHA-6]5+ is obtained with pheHA,but not picHA. The La(III)[16-MC-Zn(II),pheHA,HpheHA-6]5+ allows for Zn- Namine bond distances to be within their normal range. Instead,the geometric strain that arises from the symmetry incompatible building blocks is placed on the severely elongated Zn-Ocarbonyl bonds with the HpheHA ligand (2.34Å ). The negative charge on hydroxamates is delocalized between the Ooxime and Ocarbonyl atoms. Likely,greater charge placed on the Ooxime reduces the penalty for such a long Zn-Ocarbonyl bond distance. The La(III) [16-MC-Zn(II),pheHA,HpheHA-6]5+ structure therefore accommodates the strong Zn-Namine bonds expected for pheHA,while the M(N)[12-MCZn(II),picHA-4]N+ and Ln(III)[15-MCZn(II),picHA-5]3+ places the strain on the weaker Zn-Npyridyl bonds. Importantly,this finding presents a new ligand criterion for designing traditional high-symmetry MCs with symmetry incompatible building blocks. Weak donor groups at the α-carbon of the hydroximate ligand favor high-symmetry MCs by accommodating the geometric strain at a weakly bound site.
PheHA assembles fewer MC species than picHA based on the ESI-MS results. A number of complexes observed with picHA were not formed with pheHA,such as the LnZn16(picHA)16 3+ assembled in methanol,LnZn8(picHA)83+ assembled in water/pyridine,and LnZn12(picHA)123+ assembled in DMF [25]. The absence of LnZn12(pheHA)123+ and LnZn16(pheHA)163+ is likely a steric issue. The phenyl side chain makes pheHA bulkier than picHA. There is likely difficulty in accommodating the phenyl side chain in the [24-MCZn(II),pheHA-8] due to the close proximity of the Ln(III) [12-MCZn(II),pheHA-4]3+ in these complexes. One must also consider that MC assembly competes with low nuclearity complexes, particularly mononuclear complexes [30, 31]. We have encountered significant difficulty in observing these complexes by ESI-MS, so their presence must be assumed. Nevertheless,the low nuclearity Zn-pheHA complexes may be stronger competitors for the formation of MCs than the analogous Zn-picHA complexes depending on the solvent conditions. 4. Conclusion
Metal ion substitution is an attractive strategy for designing functional coordination macrocycles,though changes in the metal ion coordination geometry can drastically impact the assembly process. We have now demonstrated with two different ligands that substituting Zn(II) for Cu(II) or Ni(II) can drastically impact the assembly of the prototypical Ln(III)[15-MCM(II),L-5]3+. Solvent dependent assembly is evident in the previously studied picHA ligand and now with an α-aminoHA as well,solidifying the link between symmetry incompatible building blocks and the assembly of multiple complexes through solvent dependent behavior. The first [16-MC-6] was crystallized that unusually possesses C2-symmetry and a mixture of singly-deprotonated and doubly deprotonated pheHA ligands. This complex suggests that weakly basic ligands on the α-carbon may favor the assembly of high symmetry MCs by accommodating the resulting geometric strain in the complex.
AcknowledgmentsThis research was supported in part by the National Science Foundation under grant CHE-1057331. Crystallographic data is available from the Cambridge Crystallographic Data Center using deposition number 1035174.
| [1] | G. Mezei, C.M. Zaleski, V.L. Pecoraro, Structural and functional evolution of metallacrowns, Chem. Rev. 107 (2007) 4933-5003. |
| [2] | V.L. Pecoraro, A.J. Stemmler, B.R. Gibney, et al., Metallacrowns: a new class of molecular recognition agents, Prog. Inorg. Chem. 45 (1997) 83-177. |
| [3] | C.M. Zaleski, E.C. Depperman, J.W. Kampf, M.L. Kirk, V.L. Pecoraro, Synthesis, structure, and magnetic properties of a large lanthanide-transition-metal singlemolecule magnet, Angew. Chem. Int. Ed. 43 (2004) 3912-3914. |
| [4] | C.M. Zaleski, J.W. Kampf, T. Mallah, M.L. Kirk, V.L. Pecoraro, Assessing the slow magnetic relaxation behavior of LnIII4MnIII6 metallacrowns, Inorg. Chem. 46 (2007) 1954-1956. |
| [5] | C.M. Zaleski, S. Tricard, E.C. Depperman, et al., Single molecule magnet behavior of a pentanuclear Mn-based metallacrown complex: solid state and solution magnetic studies, Inorg. Chem. 50 (2011) 11348-11352. |
| [6] | T.T. Boron, J.W. Kampf, V.L. Pecoraro, A mixed 3d-4f 14-metallacrown-5 complex that displays slow magnetic relaxation through geometric control of magnetoanisotropy, Inorg. Chem. 49 (2010) 9104-9106. |
| [7] | C.M. Zaleski, E.C. Depperman, J.W. Kampf, M.L. Kirk, V.L. Pecoraro, Using LnIII[15-MCCuII(N)(S)-pheHA-5]3+ complexes to construct chiral single-molecule magnets and chains of single-molecule magnets, Inorg. Chem. 45 (2006) 10022-10024. |
| [8] | J. Jankolovits, C.M. Andolina, J.W. Kampf, K.N. Raymond, V.L. Pecoraro, Assembly of near-infrared luminescent lanthanide host (host-guest) complexes with a metallacrown sandwich motif, Angew. Chem. Int. Ed. 50 (2011) 9660-9664. |
| [9] | E.R. Trivedi, S.V. Eliseeva, J. Jankolovits, et al., Highly emitting near-infrared lanthanide "encapsulated sandwich" metallacrown complexes with excitation shifted toward lower energy, J. Am. Chem. Soc. 136 (2014) 1526-1534. |
| [10] | M. Moon, I. Kim, M.S. Lah, Three-dimensional framework constructed using nanometer-sized metallamacrocycle as a secondary building unit, Inorg. Chem. 39 (2000) 2710-2711. |
| [11] | C.S. Lim, J. Jankolovits, J.W. Kampf, V.L. Pecoraro, Chiral metallacrown supramolecular compartments that template nanochannels: self-assembly and guest absorption, Chem. Asian J. 5 (2010) 46-49. |
| [12] | A.V. Pavlishchuk, S.V. Kolotilov, M. Zeller, et al., Magnetic and sorption properties of supramolecular systems based on pentanuclear copper(Ⅱ) 12-metallacrown-4 complexes and isomeric phthalates: structural modeling of the different stages of alcohol sorption, Eur. J. Inorg. Chem. 2011 (2011) 4826-4836. |
| [13] | J.T. Grant, J. Jankolovits, V.L. Pecoraro, Enhanced guest affinity and enantioselectivity through variation of the Gd3+[15-Metallacrown-5] side chain, Inorg. Chem. 51 (2012) 8034-8041. |
| [14] | M. Tegoni, M. Tropiano, L. Marchio, Thermodynamics of binding of carboxylates to amphiphilic Eu3+/Cu2+ metallacrown, Dalton Trans. (2009) 6705-6708. |
| [15] | M. Tegoni, M. Remelli, Metallacrowns of copper(Ⅱ) and aminohydroxamates: thermodynamics of self assembly and host-guest equilibria, Coord. Chem. Rev. 256 (2012) 289-315. |
| [16] | A.D. Cutland, J.A. Halfen, J.W. Kampf, V.L. Pecoraro, Chiral 15-metallacrown-5 complexes differentially bind carboxylate anions, J. Am. Chem. Soc. 123 (2001) 6211-6212. |
| [17] | J.C.-G. Bunzli, C. Piguet, Lanthanide-containing molecular and supramolecular polymetallic functional assemblies, Chem. Rev. 102 (2002) 1897-1928. |
| [18] | N. Sabbatini, S. Perathoner, G. Lattanzi, S. Dellonte, V. Balzani, Electron- and energy-transfer processes involving excited states of lanthanide complexes: evidence for Inner-sphere and Outer-sphere mechanisms, Inorg. Chem. 27 (1988) 1628-1633. |
| [19] | A.J. Stemmler, J.W. Kampf, M.L. Kirk, B.H. Atasi, V.L. Pecoraro, The preparation, characterization, and magnetism of copper 15-metallacrown-5 lanthanide complexes, Inorg. Chem. 38 (1999) 2807-2817. |
| [20] | M. Tegoni, M. Furlotti, M. Tropiano, C.-S. Lim, V.L. Pecoraro, Thermodynamics of core metal replacement and self-assembly of Ca2+ 15-metallacrown-5, Inorg. Chem. 49 (2010) 5190-5201. |
| [21] | F. Dallavalle, M. Remelli, F. Sansone, D. Bacco, M. Tegoni, Thermodynamics of selfassembly of copper(Ⅱ) 15-metallacrown-5 of Eu(III) or Gd(III) with (S)-a-Alaninehydroxamic acid in aqueous solution, Inorg. Chem. 49 (2010) 1761-1772. |
| [22] | S.H. Seda, J. Janczak, J. Lisowski, Synthesis and structural characterisation of nickel 15-metallacrown-5 complexes with lanthanide(III) and lead(Ⅱ) ions: influence of the central metal ion size on the spin state of peripheral nickel(Ⅱ) ions, Inorg. Chem. Commun. 9 (2006) 792-796. |
| [23] | Metallacrown nomenclature follows the formula M(N)[# ring atoms-MCM0(N0), L-# ring oxygens](anions) (coordinated ligands) where M is the central ion, N is the oxidation state of the central metal, MC is the abbreviation of metallacrown, M0 is the ring ion, N0 is the oxidation state of the ring ion, and L is the ligand. |
| [24] | J. Jankolovits, J.W. Kampf, V.L. Pecoraro, Insight into the structural versatility of the Ln(III)[15-metallacrown-5] platform by comparing analogs with Ni(Ⅱ), Cu(Ⅱ), and Zn(Ⅱ) ring ions, Polyhedron 52 (2013) 491-499. |
| [25] | J. Jankolovits, J.W. Kampf, V.L. Pecoraro, Solvent dependent assembly of lanthanide metallacrowns using building blocks with incompatible symmetry preferences, Inorg. Chem. 53 (2014) 7534-7546. |
| [26] | A.J. Stemmler, J.W. Kampf, V.L. Pecoraro, A planar[15]metallacrown-5 that selectively binds the uranyl cation, Angew. Chem. Int. Ed. Engl. 35 (1996) 2841-2843. |
| [27] | R. Chakrabarty, P.S. Mukherjee, P.J. Stang, Supramolecular coordination: selfassembly of finite two- and three-dimensional ensembles, Chem. Rev. 111 (2011) 6810-6918. |
| [28] | A.J. Stemmler, J.W. Kampf, V.L. Pecoraro, Synthesis and crystal structure of the first inverse 12-metallacrown-4, Inorg. Chem. 34 (1995) 2271-2272. |
| [29] | M. Careri, F. Dallavalle, M. Tegoni, I. Zagnoni, Pentacopper(Ⅱ) 12-metallacrown-4 complexes with a- and b-aminohydroxamic acids in aqueous solution: a reinvestigation, J. Inorg. Biochem. 93 (2003) 174-180. |
| [30] | M. Tegoni, M. Remelli, D. Bacco, L. Marchio, F. Dallavalle, Copper(Ⅱ) 12-metallacrown- 4 complexes of a-, b- and g-aminohydroxamic acids: a comparative thermodynamic study in aqueous solution, Dalton Trans. (2008) 2693-2701. |
| [31] | L. Marchio, N. Marchetti, C. Atzeri, V. Borghesani, M. Remelli, M. Tegoni, The peculiar behavior of Picha in the formation of metallacrown complexes with Cu(Ⅱ), Ni(Ⅱ) and Zn(Ⅱ) in aqueous solution, Dalton Trans. 44 (2015) 3237-3250. |
| [32] | D. Bacco, V. Bertolasi, F. Dallavalle, et al., Metallacrowns of Ni(Ⅱ) with a-aminohydroxamic acids in aqueous solution: beyond a 12-MC-4, an unexpected (vacant?) 15-MC-5, Dalton Trans. 40 (2011) 2491-2501. |
| [33] | J. Jankolovits, J.W. Kampf, V.L. Pecoraro, Isolation of elusive tetranuclear and pentanuclear M(Ⅱ)-hydroximate intermediates in the assembly of lanthanide [15-metallacrown-5] complexes, Inorg. Chem. 52 (2013) 5063-5076. |
| [34] | J. Jankolovits, C.-S. Lim, G. Mezei, J.W. Kampf, V.L. Pecoraro, Influencing the size and anion selectivity of dimeric Ln3+[15-metallacrown-5] compartments through systematic variation of the host side chains and central metal, Inorg. Chem. 51 (2012) 4527-4538. |
| [35] | Bruker Analytical X-ray, Saint Plus v 7.60, Madison, WI, 2009. |
| [36] | G.M. Sheldrick, Program for Empirical Absorbtion Correction of Area Detector Data, Gottingen, Germany, 2008. |
| [37] | G.M. Sheldrick, A short history of SHELX, Acta Crystallogr. A64 (2008) 112-122. |
| [38] | F. Dallavalle, M. Tegoni, Speciation and structure of copper(Ⅱ) complexes with (s)- phenylalanine- and (s)-tryptophanhydroxamic acids in methanol/water solution: a combined potentiometric, spectrophotometric, CD and ESI-MS study, Polyhedron 20 (2001) 2697-2704. |
| [39] | M. Tegoni, F. Dallavalle, B. Belosi, M. Remelli, Unexpected formation of a copper(Ⅱ) 12-metallacrown-4 with (S)-glutamic-gamma-hydroxamic acid: a thermodynamic and spectroscopic study in aqueous solution, Dalton Trans. (2004) 1329-1333. |
| [40] | M. Remelli, D. Bacco, F. Dallavalle, et al., Stoichiometric diversity of Ni(Ⅱ) metallacrowns with b-alaninehydroxamic acid in aqueous solution, Dalton Trans. 42 (2013) 8018-8025. |
| [41] | M. Tegoni, L. Ferretti, F. Sansone, et al., Synthesis, solution thermodynamics, and X-ray study of CuII [12]metallacrown-4 with GABA hydroxamic acid: an unprecedented crystal structure of a [12]MC-4 with a g-Aminohydroxamate, Chem. Eur. J. 13 (2007) 1300-1308. |
| [42] | B. Kurzak, E. Farkas, T. Glowiak, H. Kozlowski, J. Chem. Soc. Dalton Trans. (1991) 163-167. |
| [43] | M.S. Lah, V.L. Pecoraro, Isolation and characterization of {MnII[MnIII(salicylhydroximate)] 4(acetate)2(DMF)6}.cntdot.2DMF: an inorganic analog of M2+(12- crown-4), J. Am. Chem. Soc. 111 (1989) 7258-7259. |
| [44] | B.R. Gibney, D.P. Kessissoglou, J.W. Kampf, V.L. Pecoraro, Copper(Ⅱ) 12-metallacrown- 4: synthesis, structure, ligand variability, and solution dynamics in the 12-MC-4 structural motif, Inorg. Chem. 33 (1994) 4840-4849. |
| [45] | A.J. Stemmler, A. Barwinski, M.J. Baldwin, V. Young, V.L. Pecoraro, Facile preparation of face differentiated, chiral 15-metallacrown-5 complexes, J. Am. Chem. Soc. 118 (1996) 11962-11963. |
| [46] | J.A. Halfen, J.J. Bodwin, V.L. Pecoraro, Preparation and characterization of chiral copper 12-metallacrown-4 complexes, inorganic analogues of tetraphenylporphyrinatocopper( II), Inorg. Chem. 37 (1998) 5416-5417. |
| [47] | R. Codd, Traversing the coordination chemistry and chemical biology of hydroxamic acids, Coord. Chem. Rev. 252 (2008) 1387-1408. |
| [48] | A.W. Addison, T.N. Rao, J. Reedijk, J. van Rijn, G.C. Verschoor, Synthesis, structure, and spectroscopic properties of copper(Ⅱ) compounds containing nitrogen-sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-20-yl)-2,6-dithiaheptane]copper(Ⅱ) perchlorate, J. Chem. Soc. Dalton Trans. (1984) 1349-1356. |
| [49] | M.K. Sharma, P. Lama, P.K. Bharadwaj, Reversible single-crystal to single-crystal exchange of guests in a seven-fold interpenetrated diamondoid coordination polymer, Cryst. Growth Des. 11 (2011) 1411-1416. |
| [50] | B.J. Holliday, C.A. Mirkin, Strategies for the construction of supramolecular compounds through coordination chemistry, Angew. Chem. Int. Ed. 40 (2001) 2022-2043. |




