In the body,drugs are transported in the blood where they can encounter over 100,000 proteins. The vast majority of these proteins are albumin (55%) and immunoglobulins (38%),such as IgG,IgA,and IgM,with smaller amounts of lipoproteins and transferrin [1]. All drugs or drug delivery scaffolds come into contact with these proteins,and the complexes formed often dominate the observed pharmacokinetics and biodistribution. These protein-drug interactions have long played a significant role in small molecule drug design and are now recognized to greatly complicate the development of new drug delivery scaffolds in the field of nanomedicine [2]. One solution to this challenge is the judicious selection of an endogenous serum protein as the delivery scaffold for a given drug,imaging agent,or theranostic combining therapy and imaging [3]. Of the serum proteins,albumin has garnered the most attention and resulted in clinical applications [4, 5, 6]. There are currently seven clinically approved drugs or imaging agents employing the albumin scaffold,with applications including the treatment of metastatic breast cancer (Abraxane®) and diabetes (Levemir®,Victoza®) and imaging of cardiovascular and cerebral circulation (99mTc-Albures,Vasovist®) and lymph nodes (99mTc-Nanocol). Albumin is currently being explored for a variety of other applications,including theranostics [7, 8]. Transferrin has also been explored for drug and imaging agent delivery;however,transferrin-based systems have yet to reach the clinic [9]. Both albumin (66.5 kDa) and transferrin (78 kDa) have molecular weights above the renal clearance threshold,contributing to long circulation times. Both proteins accumulate in malignant and inflamed tissue due to the enhanced permeation and retention (EPR) effect and internalize into cells via receptorspecific endocytosis processes. These favorable properties,and the successes noted above,have prompted extensive research into both of these proteins,with over 4000 papers published to date.
Despite these successes,the toxicity of small molecule cancer therapeutics remains a significant challenge. Off-target dosing (uptake of the cancer therapeutic by healthy cells as well as the tumor cells) leads to a wide range of side effects,sometimes necessitating sub-optimal dosing,which can lead to worse outcomes for patients. To address this problem,researchers have worked to develop targeted therapeutics that deliver drug to tumor cells while avoiding healthy cells. Folic acid (FA) is a widely studied targeting ligand for both molecular and nanoscale cancer therapies because folate receptors (FRs) are overexpressed on the surfaces of the cancer cell membranes [10, 11]. Folate is necessary for thymidine biosynthesis,and hence for de-novo DNA biosynthesis, and so rapidly dividing cancer cells increase the concentration of FRs on plasma membrane surfaces. To date,seven FAtargeted cancer therapeutics have advanced to clinical trials,but none have progressed to full clinical development. Even with targeted drug delivery agents,dose-limiting toxicity due to uptake by healthy cells remains a problem. Additionally,the expression of FRs on the surfaces of tumor cells is highly variable both from individual to individual and within a given cancer type. The folatehowever,transferrin-based systems have yet to reach the clinic [9]. Both albumin (66.5 kDa) and transferrin (78 kDa) have molecular weights above the renal clearance threshold,contributing to long circulation times. Both proteins accumulate in malignant and inflamed tissue due to the enhanced permeation and retention (EPR) effect and internalize into cells via receptorspecific endocytosis processes. These favorable properties,and the successes noted above,have prompted extensive research into both of these proteins,with over 4000 papers published to date. Despite these successes,the toxicity of small molecule cancer therapeutics remains a significant challenge. Off-target dosing (uptake of the cancer therapeutic by healthy cells as well as the tumor cells) leads to a wide range of side effects,sometimes necessitating sub-optimal dosing,which can lead to worse outcomes for patients. To address this problem,researchers have worked to develop targeted therapeutics that deliver drug to tumor cells while avoiding healthy cells. Folic acid (FA) is a widely studied targeting ligand for both molecular and nanoscale cancer therapies because folate receptors (FRs) are overexpressed on the surfaces of the cancer cell membranes [10, 11]. Folate is necessary for thymidine biosynthesis,and hence for de-novo DNA biosynthesis, and so rapidly dividing cancer cells increase the concentration of FRs on plasma membrane surfaces. To date,seven FAtargeted cancer therapeutics have advanced to clinical trials,but none have progressed to full clinical development. Even with targeted drug delivery agents,dose-limiting toxicity due to uptake by healthy cells remains a problem. Additionally,the expression of FRs on the surfaces of tumor cells is highly variable both from individual to individual and within a given cancer type. The folate metabolic pathway is also the target for inhibitors of dihydrofolate reductase (DHFR) [12, 13, 14, 15]. Clinically approved DHFR-inhibitor drugs are used to treat a variety of cancers and autoimmune diseases (methotrexate,pemetrexed),bacterial infections (trimetrexate, piritrexim),and malaria (pyrimethamine).
Can the substantial advantages of employing an endogenous serum protein for drug delivery be combined with drugs designed to target and inhibit the folate metabolic pathway? This minireview discusses recent advances in the understanding of soluble folate binding protein (FBP) and possible applications of this protein for drug delivery. First,we review the structure and hypothesized functions for FBP,including possible roles in folate metabolism. The approaches for isolation of the protein are also discussed. Second,we examine recent data regarding the detailed binding mechanism of FBP with FA,FA-conjugates,and antifolate (aFA)-conjugates. Third,we discuss the outlook for folate binding protein as a transport agent for therapeutics and imaging agents, including advantages and challenges of this approach. 2. The structure,function,and isolation of folate binding protein
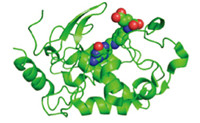
Folate binding protein (FBP) is a ~30 kDa glycoprotein containing 222 amino acids present in 1-2 nmol/L concentrations in human serum and other body fluids and 100 nmol/L concentrations in milk [16, 17, 18, 19, 20]. The functions of FBP in the body are not well understood,but it has been hypothesized to regulate the trafficking and homeostasis of folate,protect against folate degradation,and shield against bacterial utilization of folate. FBP is closely related to two isoforms of membrane-bound FRs: FR-α and FR-β,both of which are connected to plasma cell membranes via glycosylphosphatidylinositol (GPI) anchors [17, 21]. A third isoform,FR-γ,is a secreted protein and lacks the signal for modification with a GPI anchor. Soluble FBP likely originates from FR-α that has undergone cleavage of the GPI anchor and from FR-g, which inherently lacks a GPI modification. X-ray crystal structures of the FA-bound protein were recently reported (Fig. 1) [14, 15]. FBP is obtained on the gram scale by purification of whey protein [20, 22, 23, 24, 25, 26],although engineered proteins have been expressed in Chinese Hamster Ovary (CHO) cells [14].
|
Download:
|
| Fig. 1. The X-ray structure of folate receptor a with folic acid in the binding site. | |
Glycosylation of the protein is not required for the FA-binding activity of soluble FBP [27, 28, 29]. The quaternary structure of FBP changes as a function of FA-binding,consistent with a slow-onset, tight-binding interaction. At micromolar concentrations,the binding of FA to FBP also induces a self-assembly/aggregation process that has been examined in vitro [30, 31]. Interestingly,the aggregation of FR-α in the cell membrane has been shown to be an integral part of FA-binding and cellular internalization [32, 33]. Recently, it was discovered that FBP internalizes into cells via a megalin-mediated endocytosis pathway [34],suggesting the possibility of megalin playing a direct role in folate metabolism. This observation is particularly interesting for the use of FBP in chemotherapeutic targeting. 3. The binding mechanism of folic acid to folate binding protein
Folic acid binds to FBP via a slow-onset,tight-binding mechanism (Eq. (1)) [35]. The initial FBP interaction with folic acid is followed by a reorganization of the protein structure, leading to the observed nanomolar FA-FBP binding constant. The induction of structural change in the FBP upon binding FA is characterized by quenching of the inherent tryptophan fluorescence in FBP [30, 36]. The change in structure is hypothesized to lead to reduction of the number hydrophobic residues on the protein surface,resulting in a FA-ligand induced aggregation of the protein [37, 38]. At pH 7.4,the degree of aggregation (n) is dependent on FBP concentration. As measured by gel-filtration,at concentrations of 1-10 nmol/L,FBP-FA is monomeric,whereas a tetramer (FBP-FA)4 and a nonamer (FBP-FA)9,were observed for concentrations of 1.0 μmol/L and 10 μmol/L,respectively. Ultracentrifugation experiments indicated that oligomers as high as (FBP-FA)30 were present for 100 μmol/L solutions of FBP. High performance liquid chromatography (HPLC) and sodium dodecyl sulfate-poly(acrylamide) gel electrophoresis (SDS-PAGE) both indicate the formation of three new species upon FA binding to FBP [39]. The HPLC studies are particularly interesting as they provide a ready method for quantifying the relative amounts of each species present in solution. More work is needed to understand how these three species relate to tetramers,nonamers, and other species reported in the fluorescence,gel-filtration,and ultracentrifugation studies. These data indicate that a monomer structure is anticipated to be the dominant form of the protein in most biological tissues where the FBP concentration is 1-2 nmol/L; however,these reported oligomerization properties may play an important role in the binding and aggregation of FR-α in the cell membrane prior to internalization. A stopped-flow kinetic study examined the relative binding strengths of folic acid to FBP and to albumin [36]. This comparison is of particular interest since albumin has a concentration of ~0.6-0.7 μmol/L in blood,or a factor ~500,000 more concentrated than FBP. FBP binds FA tightly with a Kd < 1.3 nmol/L,whereas albumin exhibits much weaker binding with a Kd of 21 ± 2.1 μmol/L. For human plasma,this indicates that FBP will be fully bound by folate,with the remaining 7- 30 nmol/L of folate present more weakly associated with albumin. The values of Kdsuggest that about 3% of folate in human serum will be present in free form. Lowering the pH from 7.4 to the more acidic values commonly found in endosomes activates deoligomerization of the FBP and release of the bound FA.

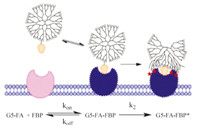
There has been a substantial amount of interest in using FAconjugates for targeted drug and imaging agent delivery [10, 11, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50] and for targeted polymer vectors [51, 52, 53, 54, 55]. Based on the FBP and albumin concentrations present in human serum [36],FA-conjugates employed at a micromolar concentration would be expected to interact extensively with albumin as well as to saturate all available soluble FBP. The rate of binding to FBP already present in serumwould be determined by the koff of existing bound FA and the production of new FBP. This suggests that both FBP and albumin could play useful roles in biodistribution of FA-conjugates; however,this aspect of their in vivo delivery has been poorly explored to date. Generally,it has been presumed that small molecule conjugates will bind with equal or lesser affinity to FBP (or FR-α) as compared to FA. HPLC and SDS-PAGE assessment of the interaction of FBP with FA-conjugates of generation 5 poly(amidoamine) (G5 PAMAM) dendrimer indicated the formation of complexes and that these complexes were stable to the addition of further free of FA [39]. It has been a goal to design polymer conjugates that have increased avidity for membrane bound FR-α due to multivalent binding [52],and surface-bound FBP has been used to model FR-α [53, 56]. The surface plasmon resonance (SPR) technique used to quantify binding in many of these studies detected an increase in binding with the number of FA attached to the polymer,as well as evidence of a tightly bound fraction that did not desorb over ~5-10 min of the experiment. This behavior was attributed to multivalency,with increasing numbers of FA giving greater avidity. Unfortunately,SPR,which is only sensitive to changes in surface mass,is unable to detect the changes in protein structure that occur during a slow-onset,tight-binding mechanism. Subsequent studies employing G5 PAMAM dendrimer containing just one FA per polymer particle exhibited the same irreversible binding to the FBP that had been ascribed to 2-4 FA conjugated to a single dendrimer multivalently binding to 2-4 FBP [57]. In addition,it was demonstrated that the increase in binding constant was proportional to total FA concentration. These data led to a closer analysis of the work of Holm,Hansen et al. and the assignment of a slow-onset,tight binding mechanism for the interaction of FA-conjugates of G5 PAMAM with FBP,as illustrated in Fig. 2. SPR binding studies of methotrexate-conjugates of G5 PAMAM monomer and dimer species were also consistent with this binding mechanism [58]. Additional experiments are needed to fully explore the nature of the polymer structure,molecular weight, and topological constraints associated with these FBP interactions. Namely,this type of binding can only be achieved if the polymer does not bind to FBP prior to the binding of FA; however,following FA-binding and the rearrangement of the protein surface,it must be favorable for a network of van der Waals interactions to form between the polymer and the protein.
|
Download:
|
| Fig. 2. Polymer-FA conjugate binding to protein. In an initial reversible step,FA binds to the protein receptor site,inducing structural change in the protein and triggering the polymer-protein interactions. In the second irreversible step,the polymer and protein form a network of van der Waals interactions leading to very strong binding. | |
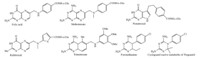
Serum binding proteins offer an important vector for the transport of drugs and imaging agents [3]. Albumin has the advantages of being the most common serum protein (0.6- 0.7 μmol/L) and is promiscuous in terms of binding to a wide range of hydrophobic molecules [3, 4, 5, 6]. It is also large enough at 66.5 kDa to avoid renal clearance. By way of contrast,FBP is present at just 1-2 nmol/L in blood and at 30 kDa is cleared by the kidney; however,these differences offer an interesting opportunity to engineer FBP for drug delivery. First,if longer circulation times are desired,the natural oligomerization mechanism of FBP provides an opportunity to form dimer,tetramers,or larger species that will be above the renal threshold for clearance. Can the degree of oligomerization be controlled by the binding strength between FBP and the FA-conjugate? The interaction with FBP provides a pathway to avoid renal clearance for a wide variety of FAconjugated materials. Indeed,such an oligomerization process may already be occurring in vivo. Second,the much lower plasma concentration of FBP may be coupled to a more specific,and more active,endocytosis mechanism than the pathway(s) employed by albumin. The recent discovery of a megalin-mediated endocytosis pathway for FBP [34] is very promising,as this could provide a route for the uptake of the FBP/FA-conjugate complexes. Again,it is possible such a pathway is already followed in vivo by FAconjugates and/or that additional studies could further develop this mechanism as an important approach to selective uptake. Third,FBP,as part of the folic acid metabolic pathway,is mechanistically linked to a highly successful class of drugs, namely the aFAs,which include methotrexate,pemetrexed, raltitrexed,trimetrexate,and pyrimethamine (Fig. 3) [12, 59]. By developing methods to strongly bind aFA-conjugates to FBP prior to injection into the bloodstream,it may be possible to increase the specificity of targeting of the antifolate drug as a chemotherapeutic agent. Like albumin,FBP oligomers should passively target the tumor via the EPR effect [3]. Then,up-regulated megalin receptors, which have been observed in the T-47D and MCF-7 breast cancer lines [60] and in prostate cancer tissue [61],can play a role in active uptake of the FBP. It has already been demonstrated that megalin up-regulation can be employed for targeted delivery using apolipoproteins [60]. Based on these literature reports,targeting of prostate and breast cancer using FBP appears to be a particularly promising area to explore.
|
Download:
|
| Fig. 3. The structures of folic acid and a variety of antifolates. | |
In addition to cancer applications,antifolates have also been employed for control of malaria. Indeed,proguanil and pyrimethamine were the drugs of choice prior to development of widespread resistance to this therapy [62, 63]. Toxicity and efficacy concerns with the artemisinin-based combination therapies have caused pyrimethamine-based therapy to remain the best option for intermittent preventative treatment for pregnant women and infants. The high-level of expression of megalin in the infant intestine suggests that FBP-based vectors may be a generally effective approach to drug delivery. Prebinding the drug to FBPmay assist in both uptake and subsequent transport of the drug. 6. Summary
Serum binding proteins show exceptional promise for drug and imaging agent delivery applications [3],and numerous successful therapeutics based on albumin are currently in the clinic [4, 5, 6]. FBP,another serum protein,is of particular interest because of its role in folate metabolism and the natural oligomerization process that occurs when FA binds to FBP. The FA-FBP interaction has been shown to proceed through a slow-onset,tight-binding mechanism leading to changes in the quaternary structure of the protein. In addition,the pH dependence of both FA-binding and oligomerization contribute to a natural release mechanism for materials inside the cell. These three facets of this protein’s behavior offer a powerful set of tools for designing the next generation drug and imaging agent delivery materials.
| [1] | Y. Shen, J.M. Jacobs, D.G. Camp, et al., Ultra-high-efficiency strong cation exchange LC/RPLC/MS/MS for high dynamic range characterization of the human plasma proteome, Anal. Chem. 76 (2004) 1134-1144. |
| [2] | I. Lynch, K.A. Dawson, Protein-nanoparticle interactions, Nano Today 3 (2008) 40-47. |
| [3] | F. Kratz, B. Elsadek, Clinical impact of serum proteins on drug delivery, J. Controlled Release 161 (2012) 429-445. |
| [4] | A.M. Merlot, D.S. Kalinowski, D.R. Richardson, Unraveling the mysteries of serum albumin-more than just a serum protein, Front. Physiol. 5 (2014) 1-7. |
| [5] | B. Elsadek, F. Kratz, Impact of albumin on drug delivery-new applications on the horizon, J Controlled Release 157 (2012) 4-28. |
| [6] | F. Kratz, Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles, J Controlled Release 132 (2008) 171-183. |
| [7] | Q. Chen, C. Liang, X. Wang, et al., An albumin-based theranostic nano-agent for dual-modal imaging guided photothermal therapy to inhibit lymphatic metastasis of cancer post surgery, Biomaterials 35 (2014) 9355-9362. |
| [8] | Q. Chen, C. Wang, Z.X. Zhan, et al., Near-infrared dye bound albumin with separated imaging and therapy wavelength channels for imaging-guided photothermal therapy, Biomaterials 35 (2014) 8206-8214. |
| [9] | A.N. Luck, A.B. Mason, Structure and dynamics of drug carriers and their interaction with cellular receptors: focus on serum transferrin, Adv. Drug Delivery Rev. 65 (2013) 1012-1019. |
| [10] | C.P. Leamon, Folate-targeted drug strategies for the treatment of cancer, Curr. Opin. Invest. Drugs 9 (2008) 1277-1286. |
| [11] | P.S. Low, W.A. Henne, D.D. Doorneweerd, Discovery and development of folicacid- based receptor targeting for Imaging and therapy of cancer and inflammatory diseases, Acc. Chem. Res. 41 (2008) 120-129. |
| [12] | I.M. Kompis, K. Islam, R.L. Then, DNA and RNA synthesis: antifolates, Chem. Rev. 105 (2005) 593-620. |
| [13] | M. Sharma, P.M.S. Chauhan, Dihydrofolate reductase as a therapeutic target for infectious diseases: opportunities and challenges, Future Med. Chem. 4 (2012) 1335-1365. |
| [14] | A.S. Wibowo, M. Singh, K.M. Reeder, et al., Structures of human folate receptors reveal biological trafficking states and diversity in folate and antifolate recognition, Proc. Natl. Acad. Sci. U.S.A. 110 (2013) 15180-15188. |
| [15] | C. Chen, J. Ke, X.E. Zhou, et al., Structural basis for molecular recognition of folic acid by folate receptors, Nature 500 (2013) 486-490. |
| [16] | J. Ghitis, Folate binding in milk, Am. J. Clin. Nutr. 20 (1967) 1-4. |
| [17] | M. Hoier-Madsen, J. Holm, S.I. Hansen, Alpha isoforms of soluble and membrane- linked folate-binding protein in human blood, Biosci. Rep. 28 (2008) 153-160. |
| [18] | A.C. Antony, Folate receptors, Annu. Rev. Nutr. 16 (1996) 501-521. |
| [19] | G.B. Henderson, Folate binding proteins, Annu. Rev. Nutr. 10 (1990). |
| [20] | J. Holm, L.N. Babol, N. Markova, A.J. Lawaetz, S.I. Hansen, The interrelationship between ligand binding and thermal unfolding of the folate binding protein. The role of self-association and pH, Biochim. Biophys. Acta-Proteins Proteomics 1844 (2014) 512-519. |
| [21] | B.A. Kamen, Folate receptors and therapeutic applications, in: A.L. Jackman, C.P. Leamon (Eds.), Targeted Drug Strategies for Cancer and Inflammation, Springer, New York, 2011. |
| [22] | L. Nygren-Babol, M. Jagerstad, Folate-binding protein in milk: a review of biochemistry, physiology, and analytical methods, Crit. Rev. Food Sci. Nutr. 52 (2012) 410-425. |
| [23] | N. Grossowicz, Purification and properties of the folate-binding protein, Methods Enzymol. 66 (1980) 690-694. |
| [24] | T. Treloar, P.A. Grieve, P.F. Nixon, One-step affinity purification of folate-binding protein, a minor whey protein, Aust. J. Dairy Technol. 59 (2000) 96. |
| [25] | I. Svendsen, B. Martin, T.G. Pedersen, et al., Isolation and characterization of the folate-binding protein from cows milk, Carlsberg Res. Commun. 44 (1979) 89-99. |
| [26] | I.B. Svendsen, S.I. Hansen, J. Holm, J. Lyngbye, The complete amino-acid-sequence of the folate-binding protein from cows milk, Carlsberg Res. Commun. 49 (1984) 123-131. |
| [27] | S.J. Roberts, M. Petropavlovskaja, K.N. Chung, C.B. Knight, P.C. Elwood, Role of individual N-linked glycosylation sites in the function and intracellular transport of the human alpha folate receptor, Arch. Biochem. Biophys. 351 (1998) 227- 235. |
| [28] | M. Ratnam, H. Marquardt, J.L. Duhring, J.H. Freisheim, Homologous membrane folate binding-proteins in human-placenta-cloning and sequence of a cDNA, Biochemistry 28 (1989) 8249-8254. |
| [29] | C.A. Luhrs, The role of glycosylation in the biosynthesis and acquisition of ligandbinding activity of the folate-binding protein in cultured Kb cells, Blood 77 (1991) 1171-1180. |
| [30] | S.W. Bruun, J. Holm, S.I. Hansen, C.M. Andersen, L. Norgaard, A chemometric analysis of ligand-induced changes in intrinsic fluorescence of folate binding protein indicates a link between altered conformational structure and physicochemical characteristics, Appl. Spectrosc. 63 (2009) 1315-1322. |
| [31] | J. Holm, A.J. Lawaetz, S.I. Hansen, Ligand binding induces a sharp decrease in hydrophobicity of folate binding protein assessed by 1-anilinonaphthalene-8- sulphonate which suppresses self-association of the hydrophobic apo-protein, Biochem. Biophys. Res. Commun. 425 (2012) 19-24. |
| [32] | E.J. Smart, C. Mineo, R.G.W. Anderson, Clustered folate receptors deliver 5- methyltetrahydrofolate to cytoplasm of MA104 cells, J. Cell Biol. 134 (1996) 1169-1177. |
| [33] | E. Moradi, D. Vllasaliu, M. Garnett, F. Falcone, S. Stolnik, Ligand density and clustering effects on endocytosis of folate modified nanoparticles, RSC Adv. 2 (2012) 3025-3033. |
| [34] | H. Birn, X.Y. Zhai, J. Holm, et al., Megalin binds and mediates cellular internalization of folate binding protein, FEBS J. 272 (2005) 4423-4430. |
| [35] | M.J. Sculley, J.F. Morrison, W.W. Cleland, Slow-binding inhibition: the general case, Biochim. Biophys. Acta 1298 (1996) 78-86. |
| [36] | U. Christensen, J. Holm, S.I. Hansen, Stopped-flow kinetic studies of the interaction of bovine folate binding protein (FBP) and folate, Biosci. Rep. 26 (2006) 291- 299. |
| [37] | S.I. Hansen, J. Holm, J. Lyngbye, T.G. Pedersen, I. Svendsen, Dependence of aggregation and ligand affinity on the concentration of the folate-binding protein from cows milk, Arch. Biochem. Biophys. 226 (1983) 636-642. |
| [38] | T.G. Pedersen, I.B. Svendsen, S.I. Hansen, J. Holm, J. Lyngbye, Aggregation of a folate-binding protein from cows milk, Carlsberg Res. Commun. 45 (1980) 161- 166. |
| [39] | X. Shi, X. Bi, T.R. Ganser, et al., HPLC Analysis of functionalized poly(amidoamine) dendrimers and the interaction between a folate-dendrimer conjugate and folate binding protein, Analyst 131 (2006) 842-848. |
| [40] | L.E. Kelderhouse, V. Chelvam, C. Wayua, et al., Development of tumor-targeted near infrared probes for fluorescence guided surgery, Bioconj. Chem. 24 (2013) 1075-1080. |
| [41] | G.M. van Dam, G. Themelis, L.M.A. Crane, et al., Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results, Nat. Med. 17 (2011), 1315-1319. |
| [42] | M.D. Kennedy, K.N. Jallad, D.H. Thompson, D. Ben-Amotz, P.S. Low, Optical imaging of metastatic tumors using a folate-targeted fluorescent probe, J. Biomed. Opt. 8 (2003) 636-641. |
| [43] | P.S. Low, S.A. Kularatne, Folate-targeted therapeutic and imaging agents for cancer, Curr. Opin. Chem. Biol. 13 (2009) 256-262. |
| [44] | Q. Chen, K.A. Li, S.H. Wen, et al., Targeted CT/MR dual mode imaging of tumors using multifunctional dendrimer-entrapped gold nanoparticles, Biomaterials 34 (2013) 5200-5209. |
| [45] | J.C. Li, L.F. Zheng, H.D. Cai, et al., Polyethyleneimine-mediated synthesis of folic acid-targeted iron oxide nanoparticles for in vivo tumor MR imaging, Biomaterials 34 (2013) 8382-8392. |
| [46] | Y. Wang, R. Guo, X. Cao, M. Shen, X. Shi, Encapsulation of 2-methoxyestradiol within multifunctional poly(amidoamine) dendrimers for targeted cancer therapy, Biomaterials 2011 (2011) 3322-3329. |
| [47] | P. Chen, J. Qin, B. Zhou, et al., Targeted tumor CT imaging using folic acid-modified PEGylated dendrimer-entrapped gold nanoparticles, Polym. Chem. 4 (2013) 4412-4424. |
| [48] | H. Lui, Y. Xu, S. Wen, et al., Targeted tumor computed tomagraphy imaging using low-generation dendrimer-stabilized gold nanoparticles, Chem. Eur. J. 19 (2013) 6409-6416. |
| [49] | S. Wen, H. Liu, H. Cai, M. Shen, X. Shi, Targeted and pH-responsive delivery of doxorubicin to cancer cells using multifunctional dendrimer-modified multiwalled carbon nanotubes, Adv. Healthc. Mater. 2 (2013) 1267-1276. |
| [50] | X.H. Liang, Y. Sun, L.S. Liu, et al., Regioselective synthesis and initial evaluation of a folate receptor targeted rhaponticin prodrug, Chin. Chem. Lett. 23 (2012) 1133- 1136. |
| [51] | A. Gabizon, A.T. Horowitz, D. Goren, et al., Targeting folate receptor with folate linked to extremities of poly(ethylene glycol)-grafted liposomes: in vitro studies, Bioconj. Chem. 10 (1999) 289-298. |
| [52] | J.F. Kukowska-Latallo, K.A. Candido, Z.Y. Cao, et al., Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer, Cancer Res. 65 (2005) 5317-5324. |
| [53] | J.E. Silpe, M. Sumit, T.P. Thomas, et al., Avidity modulation of folated-targeted multivalent dendrimers for evaluating biophysical models of cancer targeting nanoparticles, ACS Chem. Biol. 8 (2013) 2063-2071. |
| [54] | Y. Zhang, M.Y. Xu, T.K. Jiang, W.Z. Huang, J.Y. Wu, Low generational polyamidoamine dendrimers to enhance the solubiity of folic acid: a "dendritic effect" investigation, Chin. Chem. Lett. 25 (2014) 815-818. |
| [55] | S. Sunoqrot, J. Bugno, D. Lantvit, J.E. Burdette, S. Hong, Prolonged blood circulation and enhanced tumor accumulation of folate-targeted dendrimer-polymer hybrid nanoparticles, J. Controlled Release 191 (2014) 115-122. |
| [56] | S. Hong, P.R. Leroueil, I. Majoros, et al., The binding avidity of a nanoparticlebasedmultivalent targeted drug delivery platform, Chem. Biol. 14 (2007) 107- 115. |
| [57] | M.A. van Dongen, J.E. Silpe, C.A. Dougherty, et al., Avidity mechanism of dendrimer- folic acid conjugates, Mol. Pharmaceutics 11 (2014) 1696-1706. |
| [58] | M.A. van Dongen, R. Rattan, J.E. Silpe, et al., Poly(amidoamine) dendrimermethotrexate conjugates: the mechanism of interaction with folate binding protein, Mol. Pharmaceutics 11 (2014) 4049-4058. |
| [59] | J.J. McGuire, Anticancer antifolates: current status and future directions, Curr. Pharm. Des. 9 (2003) 2593-2613. |
| [60] | H.Y. Xue, H.L. Wong, Targeting megalin to enhance delivery of anti-clusterin small-interfering RNA nanomedicine to chemo-treated breast cancer, Eur. J. Pharm. Biopharm. 81 (2012) 24-32. |
| [61] | S.K. Holt, D.M. Karyadi, E.M. Kwon, et al., Association of Megalin genetic polymorphisms with prostate cancer risk and prognosis, Clin. Cancer Res. 14 (2008) 3823-3831. |
| [62] | I.B. Muller, J.E. Hyde, Antimalarial drugs: modes of action and mechanisms of parasite resistance, Future Microbiol. 5 (2010) 1857-1873. |
| [63] | M. Schlitzer, Malaria chemotherapeutics. Part 1: History of antimalarial drug development, currently used therapeutics, and drugs in clinical development, ChemMedChem 2 (2007) 944-986. |




