b Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing 100700, China
Metabonomics is the study of integrated changes inthe endogenous metabolites of living systems and their dynamic responses to both endogenous and exogenous factors [1]. The main subjects of metabonomics are lowmolecularweightmetabolites(MW< 1000 Da) Da) and their related biofluids,such as urine,plasma,or saliva. Nuclear magnetic resonance (NMR),gas chromatography-mass spectrometry (GC-MS),liquid chromatography-mass spectrometry (LC-MS),and other hyphenated technologies have been generally used for metabolomics studies [2, 3, 4]. Ultra-performance liquid chromatography (UPLC) coupled with MS,especially MS with high resolution and sensitivity,is popularly used in metabonomics [5].
Traditional Chinese medicine (TCM) has a long history and has been accepted by several academic communities and patients in China [6]. Combination taboo is one part of the TCM theory,which means that some herb medicines should not be used together in treatment,but the reason why is currently unclear. Investigations into this issue were performed from different angles,such as the herbs’ chemical composition,absorption,or metabolism bydifferent labs [7, 8, 9, 10]. Most of the research analyzes the mechanism from one point. However,because TCM is a ‘‘system-to-system’’ treatment method,it is more reasonable to use systems biology, such as metabonomics,to investigate the reason.
Wu-tou-tang,a TCM formula designed by Chinese medicine sage Zhang Zhongjing,was applied to treat rheumatoid arthritis, rheumatic arthritis,and joint pain for thousands of years. It is composed of Aconiti Radix Cocta,Ephedrae Herba,Paeoniae Radix Alba,Astragali Radix,and Glycyrrhiza Radix Preparata in the proportion of 2:3:3:3:3,of which,Aconiti Radix Cocta is the primary component. But Aconiti Radix and Pinelliae Rhizoma is one of the famous combination taboos in TCM. The objective of this study was to find the effect of adding Pinelliae Rhizoma into Wutou- tang in the treatment of adjuvant-induced arthritis (AA) rats, and further to understand the mechanism of combination taboo. Thus,an UPLC-Q-TOF-MS based metabonomics method was established to quantify the changes of the endogenous metabolites in urine of AA rats treated by Wu-tou-tang as well as Wu-tou-tang and Pinelliae Rhizoma (WP) co-decoctions.
2. ExperimentalMaterials: AconitiRadix Cocta,EphedraeHerba,Paeoniae Radix Alba, Astragali Radix,Glycyrrhiza Radix Preparata,and Pinelliae Rhizoma werepurchasedfromBeijingHuamiaoChineseMedicineEngineering Development Center (Beijing,China). Acetonitrile and formic acid (Fisher Scientific,Loughborough,UK) were HPLC-grade. Ultrapure water was produced by a Milli-Q plus (Milford,MA,USA) water purification system. Complete Freund’s adjuvant (CFA)was purchased from Chondrex,Inc (Redmond,WA,USA). Rat IL-1β and TNF-α ELLSA kits were obtained from Dakewe Biotech Co.,Ltd. (Beijing,China).
Extraction: Wu-tou-tang with a total weight of 700 g was immersed in 7 L of water for 1 h,and then heated to refluxing for 1.5 h. 5.6 L water was added for another 1.5 h refluxing after filtering. The filtered extraction solutions were combined and concentrated using a rotary evaporator at 60 8C and then lyophilized to get theWu-tou-tang extraction. Thesamemethodwas used to get three differentWP co-decoctions in which the ratios of Aconiti Radix Cocta and Pinelliae Rhizoma were 0.5:1,1:1 and 2:1,respectively.
AIA model construction and treatment: Wistar rats with weights ranged from 205 to 240 g,obtained from Experimental Animal Center of Jilin University (China),were randomly divided into 6 groups,namely healthy control group (HC),AA model group (AIA),Wu-tou-tang treated group (WTT),and WP co-decoctions treated groups including BXA 0.5:1,BXB 1:1 and BXC 2:1. Prior to initiation of experimentation,all the rats were acclimated for 7 days,and then the rats except for HC were injected 0.1 mL CFA containing 10 mg/mL dead Mycobacterium tuberculosis bacteria in the left hind footpad and the rats in HC were injected 0.1 mL saline. After 14 days of the immunization,the rats in WTT,BXA,BXB and BXC were administered intragastrically at a dose of extraction which included 1.4 g crud Aconiti Radix Cocta per kilogram per day (equal to 10 mL/kg/day) for 21 days. The rats in HC and AIA were given water intragastrically as controls. After 3 weeks administration, all the rats were sacrificed,and the blood was collected for the followed analysis and the right hind joints were excised for histologic examination.
Serum biochemical parameters: The whole blood of rat was centrifuged at 3000 ×g for 10 min at 4 8C to get the serum sample. The serum samples were stored at -80 8C. Prior to analysis,the serum samples were thawed at 4 8C. ELISA kits were applied to evaluate TNF-α and IL-1β levels in rat serums.
Histologic examination: Joint tissues were fixed in 10% neutral buffered formalin,and then decalcified for 2 weeks. Joints were processed for paraffin embedding following standard protocol. Joints sections were subsequently cut,deparaffinized,dehydrated, and stained with hematoxylin and eosin (H&E) for general evaluation.
Urine sample preparation: Samples of 24-h urine were collected on the 21st day after administration and stored at -80 8C. Prior to UPLC-MS analysis,urine samples were thawed at room temperature. After centrifuging at 10,000 rpm for 10 min,the supernatant was diluted at a ratio of 1:9 with water,and then filtered through a 0.22 μm filter membrane.
UPLC-MS conditions: Metabonomics analysis was performed on a Waters Acquity UPLC system coupled with a Q-TOF SYNAPT G2 High Definition Mass Spectrometer (HDMS) (Waters,UK). Separation was performed on a Waters ACQUITY UPLC BEH C18 Column (1.7 μm,2.1 mm × 50 mm) kept at 40 8C and at a flow rate of 0.5 mL/min. 0.1% aqueous formic acid (v/v) (A) and acetonitrile (B) were used as mobile phase. The gradient elution of B was performed as follows: 5-20% B at 0-3 min,20-40%B at 3-4 min, 40-100% B at 4-6 min,100% B at 6-7 min,100-5% B at 7-7.1 min and then kept at 5% B for 4 min. The sample inject volume was 5 μL. During the whole analysis,all the samples were maintained at 4 8C. The ESI source in both positive and negative ion mode was used in MS analysis. The source temperature was 120 8C,and desolvation gas temperature was 350 8C. Nitrogen was used as cone and desolvation gas. The flow rates of cone and desolvation gas were set at 50 L/h and 700 L/h,respectively. Capillary,cone, and extraction cone voltages were set at 3.0 kV,30 V,and 5.0 V in positive ion mode,respectively,while at 2.0 kV,30 V,and 5.0 V in negative ion mode,respectively. MS data were collected in fullscan mode in the mass range of 100-1000 Da. MSE was applied for the MS2 analyses with the low collision energy of 5 eV and the high collision energy of 25 eV-35 eV.
Data analysis: The UPLC-MS raw data were processed by MassLynx V4.1 and MarkerLynx Application Manager (Waters Corporation,Milford,USA) for peak detection and alignment. EZinfo 2.0 software was applied for principal component analysis (PCA) and orthogonal projection to latent structures squaresdiscriminant analysis (OPLS-DA). Independent sample t tests between groups were determined with PASW Statistics 18.0 software,and p < 0.05 was considered significant. Biochemical databases were used to identify potential markers,such as HMDB (http://www.hmdb.ca/),METLIN (http://metlin.scripps.edu/), Massbank (http://www.massbank.jp/) and KEGG (http://www. kegg.com/).
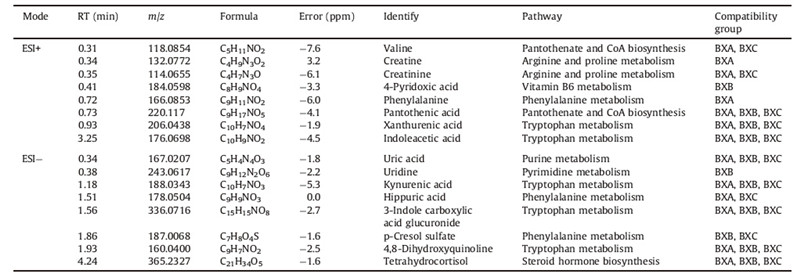
3. Results and discussion 3.1. Basic physiological parametersAfter 35 days of the immunization,swelling percent and IL-1β and TNF-α level in serum of AIA increased significantly compared with HC (Fig. 1). The percent of swelling was calculated as follows: % swelling = (paw volume 35 days after immunization/paw volume before immunization - 1) × 100%. After the treatment, the swelling percent and concentrations of inflammatory cytokines in serum decreased. But the curative effects in BXA,BXB,and BXC were worse than WTT,and the curative effect in BXB was the worst.
|
Download:
|
| Fig. 1. Physiological parameters 35 days after the immunization,swelling percent (A),IL-1β and TNF-α level in serum (B). | |
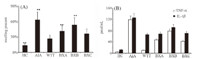
The right hind joint structures are shown in Fig. 2. These structures were characterized with the inflammatory cell infiltration, synovium proliferation,expanding synovial pannus,cartilage destruction,and bone corrosion in joints of AIA. Those mentioned pathological changes were alleviated or suppressed by administrating with Wu-tou-tang and WP co-decoctions for 3 weeks.
|
Download:
|
| Fig. 2. Histopathologic analysis of right hind joints. Original magnification 100×. | |
UPLC-Q-TOF-MS is widely used in metabonomic studies [11, 5, 12]. In this paper,both positive and negative ion modes were applied to obtain adequate information of metabolites. Because there were hundreds of metabolites detected in urine,the multivariate analysis methods,such as PCA and OPLS-DA,were used to distinguish the groups. PCA was an unbiased tool,and OPLS-DA was a supervised pattern recognition approach. Pareto scaling was used to reduce disturbances from noise and artifacts in the two models,and 7-fold cross-validation was used to minimizing the over-fitting. The WTT could be clearly separated from BXA,BXB,and BXC in PCA score plots in both positive and negative ion modes (Fig. 3),which indicated that the endogenous metabolite difference in urine was remarkable. An OPLS-DA model was established to maximize class discrimination. In the OPLS-DA model,variable importance in the projection (VIP) value was applied to find the potential biomarkers which made the greatest contribution to group separation. The ions with VIP value above 1.0 were considered as potential biomarkers. By comparing with WTT, 16 endogenous metabolites in urine were considered to be potential biomarkers that correlated with how combination influenced the therapeutic effects made by Wu-tou-tang. The identification of these metabolites was carried out as follows.

|
Download:
|
| Fig. 3. PCA score plots of urine metabolic profiling of WTT (■),BXA (▲),BXB (◆) and BXC (●) in positive mode (A) and negative modes (B). | |
The possible molecular formula of the potential biomarker was calculated by high-accuracy quasi-molecular ion within mass error of 10 ppm and fractional isotope abundance detected by Q-TOFMS. The structure information was obtained by searching freely accessible databases mentioned before. MS/MS fragmentation patterns also provided necessary information for the structures of biomarkers. Take the biomarker at m/z 206.0438 in positive ion mode as an example (Fig. 4). Suppose the m/z 206.0438 is [M+H]+, and the possible formula is C10H7NO4. Its major fragment ions in this work were m/z160.0392 and m/z 132.0438,which represent the fragment of [M-HCOOH+H]+ and [M-HCOOH-CO+H]+,respectively. Finally,it was identified as xanthurenic acid by comparing its quasimolecular ion and fragment ions in the accessible databases. The information of potential biomarkers is summarized in Table 1.

|
Download:
|
| Fig. 4. Tandem mass spectra and possible fragment pathways of m/z 206.0438. | |
| Table 1 Identification results of potential biomarkers. |
The relative contents of the biomarkers are shown in Fig. 5. These biomarkers could be divided into four groups. First,Wu-toutang could regulate the level of biomarker toward the health condition,but the treatment of WP co-decoctions were worse than Wu-tou-tang,for example,valine,p-cresol sulfate,phenylalanine, kynurenic acid,and 4,8-dihydroxyquinoline. Second,Wu-tou-tang could not make treatment effects in these biomarkers,and the WP co-decoctions made the levels of these biomarkers even worse, such as creatinine,xanthurenic acid,hippuric acid,and tetrahydrocortisol. Third,Wu-tou-tang could not regulate these biomarkers, but the WP co-decoctions could,such as,4-pyridoxic acid, pantothenic acid,and indoleacetic acid. Fourth,the levels of these biomarkers did not change in AA model or administration with Wu-tou-tang,but it changed when treated with WP co-decoctions, for example,uric acid and uridine. The first and the second kind of biomarkers showed that combination taboo made the treatment effects weaken or the AA physical condition worse. Valine and phenylalanine are two of the essential amino acids which cannot be synthesized by body. Amino acids are substrates for protein synthesis,energy metabolism, gluconeogenesis and ketogenesis. It indicated that protein synthesis or degradation might change,and biological functions such as inflammatory and autoimmunity responses might also change [13]. p-Cresol sulfate and hippuric acid are downstream products of phenylalanine,their changes indicate that combination taboo partly blocked the regulation of phenylalanine metabolism made by Wu-tou-tang. Xanthurenic acid,kynurenic acid,and 4,8-dihydroxyquinoline are downstream metabolites of tryptophan through the kynurenine pathway. Many researches showed that metabolism of tryptophan through the kynurenine pathway was related to immune system and inflammation. Some enzymes in the tryptophan metabolism such as indoleamine dioxygenase would be induced by prostaglandins and interferon when some tissues were invaded by viruses, bacteria or endotoxins [14]. Ma´ndi showed that there was a close connection between cytokines and the kynurenine system,the tryptophan metabolic pathway was activated by pro-inflammatory stimuli,and the anti-inflammatory effect of kynurenic acid provided a further feedback mechanism in modulating the immune responses [15]. Thus,the changes of kynurenine pathway may be the main reason that the treatment effects were reduced when Pinelliae Rhizoma was added in Wu-tou-tang. The fourth kind of biomarkers showed that combination taboo also made some side effects. Uric acid,the final oxidation product of purine metabolism,contributed to oxidative injury in vivo and protect against free-radical damage of DNA [16, 17]. Uridine is a nucleoside, the change of uridine indicates that the pyrimidine metabolism was disturbed. The perturbation to nucleotide metabolism might be a side effect generated by Aconiti Radix and Pinelliae Rhizoma. Though combination taboo could also benefit some biomarkers like 4-pyridoxic acid,the side effects and reduction of the treatment effects were more obvious when Pinelliae Rhizoma was added in Wu-tou-tang.

|
Download:
|
| Fig. 5. Relative content of biomarkers,compared with WTT *p < 0.05,**p < 0.01. | |
Wu-tou-tang and WP co-decoctions could decrease the swelling percent,decline the concentration of IL-1β and TNF-α in serum in AA rats,and could also alleviate inflammatory pathological changes. But the treatment effect was reduced when Pinelliae Rhizoma was added in Wu-tou-tang. UPLC-Q-TOF-MSbased metabonomics was established to quantify the changes of the endogenous metabolites in urine of AA rats treated by Wu-toutang and WP co-decoctions. There was a clear separation between Wu-tou-tang and WP decoctions treated groups in PCA model. 16 potential biomarkers had been identified for explaining the mechanism of Pinelliae Rhizoma influence on the therapeutic effects of Wu-tou-tang. Results show that for most biomarkers the regulation was heading in the opposite direction when Pinelliae Rhizoma was added into Wu-tou-tang,and combination taboo might make some side effects. This study demonstrates thatmetabonomics is a powerful methodology to gain further understanding of combination taboo.
AcknowledgmentThis research was supported by the National Basic Research Program of China (973 Program,Nos. 2011CB505300, 2011CB505305).
| [1] | J.K. Nicholson, I.D. Wilson, Understanding ‘global' systems biology: metabonomics and the continuum of metabolism, Nat. Rev. Drug Discov. 2 (2003) 668-676. |
| [2] | K. Bando, T. Kunimatsu, J. Sakai, et al., GC-MS-based metabolomics reveals mechanism of action for hydrazine induced hepatotoxicity in rats, J. Appl. Toxicol. 31 (2011) 524-535. |
| [3] | Z. Chen, S.H. Tu, Y.H. Hu, et al., Prediction of response of collagen-induced arthritis rats to methotrexate: an 1H-NMR-based urine metabolomic analysis, J. Huazhong Univ. Sci. Technol. Med. Sci. 32 (2012) 438-443. |
| [4] | Z.L. An, Y.H. Chen, R.P. Zhang, et al., Integrated ionization approach for RRLC-MS/MS-based metabonomics: finding potential biomarkers for lung cancer, J. Proteome Res. 9 (2010) 4071-4081. |
| [5] | E.M. Lenz, R.E. Williams, J. Sidaway, et al., The application of microbore UPLC/oa-TOF-MS and H-1 NMR spectroscopy to the metabonomic analysis of rat urine following the intravenous administration of pravastatin, J. Pharm. Biomed. Anal. 44 (2007) 845-852. |
| [6] | Y.M. Lao, J.G. Jiang, L. Yan, Application of metabonomic analytical techniques in the modernization and toxicology research of traditional Chinese medicine, Br. J. Pharmacol. 157 (2009) 1128-1141. |
| [7] | T.J. Han, F.R. Song, Z.Y. Liu, et al., Studies of the intestinal transport of the diterpenoid alkaloids in the aconitum carmichaeli combined with different medicinal herbs in a Caco-2 cell culture system with UPLC/MS, Acta Chim. Sin. 69 (2011) 1795-1802. |
| [8] | C.R. Xiao, Y.G. Wang, F.G. Dai, et al., The effect of aconitum co-administration with lilac daphne on cytochrome P450 in rat liver, China J. Exp. Tradit. Med. Formulae 12 (2006) 48-50. |
| [9] | F. Li, Y.G. Wang, L. Yang, et al., Regularity of toxic alkaloids during the combination of Veratrum nigrum and Salvia miltiorrhiza by UPLC-Q-TOF/MS, Acta Chim. Sin. 70 (2012) 2257-2264. |
| [10] | W.L. Liu, F.R. Song, Z.Q. Liu, et al., Chemical study on combination taboo of radix aconiti with rhizoma pinelliae, fructus trichosanthis, bulbus fritillariae thunbergli, radix ampelopsis and rhizoma bletillae, Acta Chim. Sin. 68 (2010) 889-896. |
| [11] | S.J. Bruce, I. Tavazzi, V. Parisod, et al., Investigation of human blood plasma sample preparation for performing metabolomics using ultrahigh performance liquid chromatography/mass spectrometry, Anal. Chem. 81 (2009) 3285-3296. |
| [12] | E.J. Want, I.D. Wilson, H. Gika, et al., Global metabolic profiling procedures for urine using UPLC-MS, Nat. Protoc. 5 (2010) 1005-1018. |
| [13] | X. Ouyang, Y. Dai, J.L. Wen, L. Wang, 1H NMR-based metabolomic study of metabolic profiling for systemic lupus erythematosus, Lupus 20 (2011) 1411-1420. |
| [14] | G. Allegri, C.V.L. Costa, A. Bertazzo, M. Biasiolo, E. Ragazzi, Enzyme activities of tryptophan metabolism along the kynurenine pathway in various species of animals, Farmaco 58 (2003) 829-836. |
| [15] | Y. Mándi, L. Vécsei, The kynurenine system and immunoregulation, J. Neural Transm. 119 (2012) 197-209. |
| [16] | G.K. Glantzounis, E.C. Tsimoyiannis, A.M. Kappas, D. Galaris, Uric acid and oxidative stress, Curr. Pharm. Des. 11 (2005) 4145-4151. |
| [17] | R.C. Yue, L. Zhao, Y.H. Hu, et al., Metabolomic study of collagen-induced arthritis in rats and the interventional effects of Huang-Lian-Jie-Du-Tang, a traditional Chinese medicine, Evid. Based Complement. Alternat. Med. 2013 (2013) 439690. |