Research on the solid state phase transitions triggered by temperature is of great interest for exploring novel material with interesting physical properties and understanding theoretically the relationship between structure and property [1, 2, 3]. Now phase transition materials as the data-storage applications candidate are already used in rewriteable optical data storage and offer great potential as an emerging non-volatile electronic memory [4,5,6]. Especially,a variety of ferroelectrics as the typical phase transition feature were discoveredandfound their wideapplications (are multifunctional electro-active materials with a wide range of applications,such as temperature sensing,data storage,mechanical actuation,energy harvesting and manipulate electromagnetic waves),the structure-property relationship and ferroelectric theories have been also developed and constructed [7, 8, 9, 10, 11]. The most important step for discovering the high temperature or room temperaturemolecule-based ferroelectrics is designing or searching for the high temperature phase transition compounds [12,13,14,15]. Moreover,the introduction of molecular motions to solid state material is one of the effective methods to construct the order- disorder type phase transition compounds [16, 17, 18, 19, 20, 21]. Generally, plastic crystalline states such as hexamethylethane, cyclohexane,adamantane,and fullerene (C60),whose structures are flexible, have a tendency to lead to the phase transition triggered by temperature [22, 23, 24, 25]. Among them,cyclohexane and long-chain amine with flexible structure and complicated conformation will be the good candidate group to construct the phase transition compound. Herein,we report the temperature-dependent structures and dielectric properties of the compounds with both cyclohexane and long-chain amine groups,where one of the compounds shows the high temperature phase transition owing to the flexible structure of cation.
In this paper,we present structural property and the property of the compounds. The 3-morpholin-4-yl-propyl ammonium tetrafluoroborate (MPA+) (BF4- ) (1) and the 3-morpholin-4-yl-propyl ammonium perchlorate (MPA+) (ClO4 -) (2). The compound 1 has a high temperature phase transition with the change of the space group,due to the disorder of BF4 - anion. It is a pity that the compound 2 has no phase transition.
2. Experimental 2.1. Materials and measurementsAll reagents and solvents were commercially available and used as received without further purification. The single crystal structures were judged by Rigaku SCX mini diffractometer at low,room and high temperature. Dielectric measurements were performed on pure crystalline powder of the title compound using automatic impedance TongHui2828 Analyzer. DSC measurements were performed using a Perkin-Elmer Diamond DSC instrument at the heating and cooling rate of 10 K min-1.
2.2. Synthesis(MPA+) (BF4-) and (MPA+) (ClO4-) (MPA+ is 3-morpholin-4-ylpropyl- ammonium) are offered in high yield by mixing the equimolal acid (HBF4-H2O (40%,w/w) or HClO4-H2O (70%,w/w)) and the flexible 3-morpholin-4-yl-propyl amine in the ethanol solvent. The FT-IR spectra confirm the successful syntheses of compounds 1 and 2 (Fig. S1 in Supporting information). The colorless block single crystals suitable for the X-ray crystal structural analyses were grown at room temperature.
2.3. Single-crystal X-ray diffraction measurementsSingle-crystal data were collected at 300 K and 373 K on a Rigaku SCXmini CCD diffractometer equipped with graphitemonochromated MoKa radiation. The structures were solved using direct methods and successive Fourier difference synthesis (SHELXS-97) [26],and refined using the full-matrix least-squares method on F2 with anisotropic thermal parameters (SHELXL-97) [27].
3. Results and discussionIt is well known that there have a lot of measurements can be used as the evidence of the phase transition,this is because that the physical properties like dielectric property and magnetic property may change. It is also worth to note the magnitude of this change is related to the characteristic of the phase transition.
In these measurements,DSC measurement is one of the most effective methods to detect if this compound goes through a reversible phase transition triggered by temperature in term of heat anomalies occurred during in the heating and cooling processes. The pure single crystals of 1 and 2 were used directly to measure the DSC below their melting points to check whether the phase transition occurred with the temperature changing. The melting point and the melting enthalpy of compound 1 are 418 K and -220.8 J K-1 mol-1 ,the melting point and the melting enthalpy of compound 2 are 453 K and -1792.8 J K-1 mol-1(Fig. S2 in Supporting information). Interestingly,the high temperature phase transition of compound 1 has been investigated by DSC, where the thermodynamic anomalies occurred during the heating and cooling curve. DSC curve (Fig. 1) of compound 1 shows an obvious λ-type endothermic peak at 363.4 K and an exothermic peak at 347.3 K. The reversible phase transition with a large hysteresis with the value of 16.1 K means a first-order phase transition occurred with the temperature changing, while the sharp peaks reveal the discontinuous character of the transition as shown in Fig. 1 [28, 29]. The entropy change (△S) of the phase transition is estimated from the DSC to be 5.24 J K-1 mol-1. From the Boltzmann equation,the entropy change of phase transition △S = RlnN,where N represents the ratio of possible configurations and R is the gas constant. It is found that N = 1.88,larger than 1, suggesting that the phase transition is an ordered-disordered type. Unfortunately,no thermodynamic anomalies have been found in the DSC measurement results of compound 2 from room temperature to 430 K as shown in Fig. S3 in Supporting information,indicating no phase transition occurred with the temperature changing.

|
Download:
|
| Fig. 1. DSC measurement result of the compound 1 change for DSC traces of 10 K min-1. | |
The variable temperature dielectric response is another common method of studying phase transitions,especially for high frequency range,is often useful for searching for phase transitions. Therefore,the temperature dependence of dielectric constant taken at 1 MHz for the compound 1 is depicted in Fig. 2. The temperature dependent dielectric constant of compound 1 at 1 MHz frequency has been investigated from room temperature to its melting point to confirm the high temperature phase transition. As shown in Fig. 2,a λ-type dielectric constanttemperature curve is found in the temperature-dependent dielectric constant of compound 1. With the temperature increasing the dielectric constant value change from 4.2 to 6.5 at the phase transition temperature of around 360 K,then drop from 6.5 to 3.7 after phase transition occurred,on the cooling the dielectric constant value change from 3.9 to 5.6 at the phase transition temperature of around 346 K (Fig. S4 in Supporting information). The temperature dependence of the dielectric constant measured at 5,10 and 100 KHz is shown in Fig. S5 in Supporting information,we discover that the phase transition temperatures are frequency independent. The phase transition temperature found in dielectric measurement result fits well to the DSC measurement result. The obvious dielectric anomaly is attributed to the structure phase transition and polar change in solid state compound 1. On the contrary,the temperaturedependent dielectric constant of compound 2 is almost temperature independent and has no anomaly from room temperature to its melting point,for no structure change and phase transition occurred as that found in its DSC measurement result.

|
Download:
|
| Fig. 2. The temperature dependence of the dielectric permittivity for the title compounds 1 and 2 at 1 MHz. | |
Table 1 summarizes the crystal data,data collection,and reduction parameters of compounds 1 and 2. The crystal structures of compounds 1 and 2 have been determined and analyzed at different temperature basing on the phase transition temperature. For instance,the crystal 1 is measured at 300 K and 373 K for the phase transition occurred at around 363.4 K with the temperature increasing and the structures at 300 K and 373 K correspond to the room temperature and high temperature phases,respectively. The room temperature phase structure crystallizes in a monoclinic space group of P21/c with cell parameters of a = 9.978(2) Å ,b = 7.5206(15) Å ,c = 15.058(3) Å , β = 96.99(3)8,V = 1121.6(4) Å 3,Z = 4. The high temperature structure,carried out at 373 K,is fall in the triclinic space group P-1,with cell parameters of a = 10.021(7) Å ,b = 7.662(3) Å , c = 7.920(4) Å ,α = 95.012(14)8,β = 101.67(4)8,γ = 91.48(3)8, V = 592.7(6) Å 3,Z = 2. The high temperature structure keeps a little change of a,b axis lengths compared to those in the room temperature structure due to temperature expansion effect. The approximately halving of the c axis length and volume occurred with the temperature increasing. It is important to note that the slight change of lattice angles result in the crystal system from monoclinic to triclinic as shown in Table 1. The obvious change of lattice parameters certify that the phase transition occurred from 300 K to 373 K as that found in DSCmeasurement result. As shown in Table 1,the high temperature phase structure of compound 1 (MPA+) (BF4-) shows the similar lattice data as the room temperature phase of compound 2 (MPA+) (ClO4-),so we measure the low temperature structure (100 K) of compound 2 to check whether its phase transition occurs at lower temperature. Unfortunately,no structure change and phase transition occur even the temperature decreased to 100 K as shown in Table 1. The room temperature and low temperature structures of compound 2 crystallize in the triclinic space group P-1. The low temperature structure shows slight change of axis lengths and lattice angles compared to those in the room temperature structure due to temperature contract effect.
| Table 1 Crystal data,data collection,and reduction parameter of crystals 1 and 2. |
The asymmetric unit of the compound 1 consists of an MPA+ cation and an isolate BF4- anion at both 300 K and 373 K as shown in Fig. 3. The morpholine ring of MPA+ cation adopts a chair conformation: The three non-hydrogen atoms (O1,C2 and C5) of morpholine ring lie approximately in a plane,while another three non-hydrogen atoms (N1,C3 and C4) lie in another plane to be parallel to the plane of O1-C2-C5. Different from the traditional trans-form chains of C-C-C-N [30],the C5-C6-C7-N2 group in compound 1 presents a cis-form,owing to the strong intramolecular hydrogen bond interaction of N-H···N(as shown in Table S1 in Supporting information) to form a hexatomic ring (C5-C6-C7-N2- H2A-N1).

|
Download:
|
| Fig. 3. The asymmetric structure of compound 1 at 300 K (left) and 373 K (right). | |
The most significant difference between those at 300 K and 373 K concerns the disorder of the BF4- anion. At 300 K,the BF4- anion is orientationally ordered adopting an ideally tetrahedral geometry with the B-F bond distances of 1.3004-1.3744Å and the F-B-F bond angles of 103.23-113.948,these bond lengths and angles are in good agreement with those observed in similar compounds [31]. However,at 373 K,The BF4- anion is highly orientationally disordered over two positions with occupation factors of 0.63(2) and 0.37(2) (Fig. 2). Compared with the structure of 300 K,the thermal ellipsoids of the all atoms at the 373 K are larger. Especially for C6,the equivalent isotropic displacement parameters of C6 changes from 59 to 225(7) (Fig. 3 and Table 2), meanwhile,the angle of N1-C5-C6 changes from 113.7(3) to 121.3(7)°,it is also worthwhile to note that the torsion angle of N1- C5-C6-C7,C5-C6-C7-N2 change from -65.2(4),68.5(4)° (300 K) to -36(2),38.5(17)° (373 K) (Table 3). The flexible structure of the C5-C6-C7-N2 long chain contributes to the large motion of C6 atom and the angles changes.
| Table 2 Equivalent isotropic displacement parameters for compounds 1 and 2. |
| Table 3 The selected bond distance,angle and torsion angle of compound 1. |
For the compound 1,four MPA+ cations and four BF4- anions are involved in the unit cell packing structure at 300 K,whereas there are two MPA+ cations and two BF4- anions with the temperature changing to 373 K (Fig. 4). Comparing the packing structures of 300 K with 373 K,the nearest four B atoms of BF4- form a parallelogram with the side length change from 7.3946(57), 7.7709(65) Å to 7.9200(14),7.3656(135) Å ,and the angel also change from 79.48° to 82.78° with the temperature increasing (Fig. 5). It is same to the parallelogram composed of the nearest four N atoms of NH3+ moiety from the cations,the distance between the N atoms and the angle change from 7.8381(35), 7.2450(32)Å ,88.172(32)° to 7.9200(8),7.2695(81)Å ,78.081(69)°. As a result,the small motion of cations and anions change the whole unit cell and lattice parameters. For the intermolecular hydrogen bonds,the neighboring MPA+ cations are linked by O-H···N hydrogen bonds to form one-dimensional chains along the a axis and the BF4- anions are linked to the one-dimensional MPA+ cations chains through N-H···F hydrogen bonds as shown in Fig. 6.

|
Download:
|
| Fig. 4. The packing view of compound 1 along the a axis at 300 K and 373 K. | |

|
Download:
|
| Fig. 5. The packing view of BF4-anions in compound 1 along the a axis at 300 K and 373 K. MPA+ cations have been omitted for charity. | |

|
Download:
|
| Fig. 6. The one dimensional chain H-bond interactions of compound 1 (300 K). | |
An asymmetric unit of the compound 2 at both the 300 K and the 100 K consists of an MPA+ cation and an isolate ClO4- anion and the morpholine ring of cation also adopts a chair conformation as that found in compound 1. Compare with compound 1,some bond distances and bond angles are different. For the compound 1,the distances of O-O,O-B,N-N,N-B are 7.6846(32) Å ,7.8385(35) Å , 7.8385(35) Å ,3.9369(33) Å ,the angle of O-B-N is 59.804°; to the compound 2,the distances of O-O,O-Cl,N-N,N-Cl are 7.7560(38) Å ,7.7560(2)Å ,7.7560(2)Å ,3.6384(44)Å ,and the angle of O-Cl-N is 65.078°(Fig. S6,Table 4). Maybe this is the reason why compounds 1,2 have different space group at 300 K. The similar intramolecular N-H···N hydrogen bonding interactions fix the conformation of cation. The ClO4- anion is orientationally ordered adopting an ideally tetrahedral geometry with the Cl-O bond distances and the O-Cl-O bond angles both in a reasonable range (Fig. 7 and Table 2).

|
Download:
|
| Fig. 7. The asymmetric structure of compound 2 at 300 K (left) and 100 K (right). | |
| Table 4 The selected bond distance and angle compound 1,2 (Å ,°). |
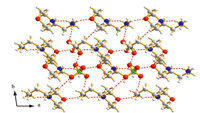
We should note that there are many intermolecular hydrogen bonds in compound 2,including N2-H2C···O1,N2-H2C···O4, N2-H2D···O4,N2-H2···O2,C2-H2B···O5,C3-H3B···O1 (Table S2 in Supporting information),and those hydrogen bonds make the compound form a two-dimensional planar structure (Fig. 8). TheH-bond supramolecular interaction should contribute to the phase transition. Unfortunately,no phase transition and structure change occurred with the temperature change as above mentioned. It is noted that the BF4- anion and the ClO4- anion involved in the formation of hydrogen bond are different: one F atom of the BF4- anion and three F atoms from another BF4- anion alternate to participate in the formation of hydrogen bond in Fig. 6; however,for the compound 2,three O atom of the every ClO4- anion are involved in the formation of hydrogen bond in Fig. 8.
|
Download:
|
| Fig. 8. The two dimensional plane H-bond interactions of compound 2 on the ab plane (100 K). | |
In summary,compounds 1 and 2 crystallize in different space group,and there packing styles and hydrogen-bonds are different at room temperature. The reason is the different of anion; this difference may be the reason why compounds 1,2 have difference in properties.
4. ConclusionIntroducing the flexible hexane ring and long chain amine is an effect method to construct phase transition compound. The temperature-dependent heat flow,structures and dielectric certified the high temperature phase transition in compound 1.
AcknowledgmentsThis work was financially supported by the Project 973 (No. 2014CB848800),National Natural Science Foundation of China (Nos. 21471032,21422101 and 21301029),Jiangsu Province NSF (Nos. BK20140056 and BK20130600),Program for NCET and Ph.D. Programs Foundation of Ministry of Education of China (No. 20130092120013).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.10.025.
| [1] | J. Zyss, Molecular Nonlinear Optics: Materials, Physics, and Devices, Academic Press, New York, 1993. |
| [2] | G.R. Desiraju, Crystal Engineering: The Design of Organic Solids, Elsevier, New York, 1989. |
| [3] | R.E. Newnham, Structure Property Relations, Springer, New York, 1975. |
| [4] | F.A. Lopez, J.M. Cabrera, F.A. Rueda, Electrooptics: Phenomena, Materials and Applications, Academic Press, New York, 1994. |
| [5] | J.L. Lehn, Supramolecular Chemistry: Concepts and Perspectives, VCH, New York, 1995. |
| [6] | M. Wuttig, N. Yamada, Phase-change materials for rewriteable data storage, Nat. Mater. 6 (2007) 824-832. |
| [7] | W. Zhang, R.G. Xiong, Ferroelectric metal-organic frameworks, Chem. Rev. 112 (2012) 1163-1195. |
| [8] | T. Hang, W. Zhang, H.Y. Ye, R.G. Xiong, Metal-organic complex ferroelectrics, Chem. Soc. Rev. 40 (2011) 3577-3598. |
| [9] | J.F. Scott, Ferroelectric Memories, Springer-Verlag, Berlin, 2000. |
| [10] | S. Horiuchi, F. Ishii, R. Kumai, et al., Ferroelectricity near room temperature in co-crystals of nonpolar organic molecules, Nat. Mater. 4 (2005) 163-166. |
| [11] | S. Horiuchi, R. Kumai, Y. Tokura, Hydrogen-bonded donor-acceptor compounds for organic ferroelectric materials, Chem. Comm. 23 (2007) 2321-2329. |
| [12] | D.W. Fu, H.L. Cai, Y.M. Liu, et al., Diisopropylammonium bromide is a hightemperature molecular ferroelectric crystal, Science 339 (2013) 425-428. |
| [13] | S. Horiuchi, Y. Tokunaga, G. Giovannetti, et al., Above-room-temperature ferroelectricity in a single-component molecular crystal, Nature 463 (2010) 789-792. |
| [14] | A.S. Tayil, A.K. Shveyd, A.C.H. Sue, et al., Room-temperature ferroelectricity in supramolecular networks of charge-transfer complexes, Nature 488 (2012) 485-489. |
| [15] | P.P. Shi, S.W. Sun, M.L. Liu, L.H. Kong, Q. Ye, Temperature-dependent crystal structures and unusual dielectric property in [Me2NH2]2[Ni(mnt)2], Inorg. Chem. Commun. 37 (2013) 84-88. |
| [16] | A. Budzianowski, A. Katrusiak, Pressure tuning between NH…N Hydrogen-Bonded ice analogue and NH…Br Polar dabcoHBr complexes, J. Phys. Chem. B. 110 (2006) 9755-9758. |
| [17] | J. Waøsicki, P. Czarnecki, Z. Pajaøk, W. Nawrocik, W. Szczepanski, Ferroelectric properties of pyridinium perrhenate, J. Chem. Phys. 107 (1997) 576-579. |
| [18] | G.C. Xu, W. Zhang, X.M. Ma, et al., Coexistence of magnetic and electric orderings in the metal-formate frameworks of [NH4][M(HCOO)3], J. Am. Chem. Soc. 133 (2011) 14948-14951. |
| [19] | Z.H. Sun, T.L. Chen, J.H. Luo, M.C. Hong, Bis(imidazolium) L-tartrate: a hydrogenbonded displacive-type molecular ferroelectric material, Angew. Chem. Int. Ed. 51 (2012) 3871-3876. |
| [20] | G.C. Xu, X.M. Ma, L. Zhang, Z.M. Wang, S. Gao, Disorder-order ferroelectric transition in the metal formate framework of [NH4][Zn(HCOO)3], J. Am. Chem. Soc. 132 (2010) 9588-9590. |
| [21] | S. Chen, R. Shang, K.L. Hu, Z.M. Wang, S. Gao, [NH2NH3][M(HCOO)3](M = Mn2+, Zn2+, Co2+ and Mg2+): structural phase transitions, prominent dielectric anomalies and negative thermal expansion, and magnetic ordering, Inorg. Chem. Front. 1 (2014) 83-98. |
| [22] | Z.H. Sun, J.H. Luo, T.L. Chen, et al., Distinct molecular motions in a switchable chromophore dielectric 4-N,N-dimethylamino-4'-N'-methylstilbazolium trifluoromethanesulfonate, Adv. Funct. Mater. 22 (2012) 4855-4861. |
| [23] | Q. Ye, T.Akutagawa, N.Hoshino, et al., Polymorphsandstructural phase transition of[Ni(dmit)2]- crystals induced by flexible (trans-cyclohexane-1,4-diammonium)(-benzo[18]crown-6)2, supramolecule, Cryst. Growth Des. 11 (2011) 4175-4182. |
| [24] | Q. Ye, T. Akutagawa, H.Y. Ye, et al., Structural phase transition due to the flexible supramolecule of (4-cyanomethylanilinium)([18]crown-6) in [Ni(dmit)2]-crystal, Cryst. Eng. Comm. 13 (2011) 6185-6191. |
| [25] | Q. Ye, P.P. Shi, X.Q. Fu, T. Akutagawa, T. Nakamura, Dielectric and structural phase transition of [Ni(dmit)2]2 salt with (4-ethoxyanilinium)([18]crown-6) supramolecular cation, Cryst. Eng. Comm. 15 (2013) 5307-5313. |
| [26] | G.M. Sheldrick, SHELXS-97, in: Program for Crystal Structure Solution, University of Gôingen, Germany, 1997. |
| [27] | A. Altomare, G. Cascarano, C. Giacovazzo, et al., SIR92-a c by direct methods, J. Appl. Cryst. 27 (1994) 435. |
| [28] | Y.Y. Tang, C.M. Ji, Z.H. Sun, et al., Phase transition originating from order-disorder transformations of carboxy oxygen atoms coupled with dynamic proton motions in [PhCH2NH(CH3)2]2C2O4·H2C2O4, Chem. Asin. J. 9 (2014) 1771-1776. |
| [29] | C.M. Ji, Z.H. Sun, S.Q. Zhang, et al., N-Isopropylbenzylammonium tetrafluoroborate: an organic dielectric relaxor with a tunable transition between high and low dielectric states, J. Mater. Chem. C 2 (2014) 567-572. |
| [30] | N. Shibata, E. Suzuki, T. Asahi, M. Shiro, Enantioselective fluorination mediated by cinchona alkaloid derivatives/selective combinations: reaction scope and structural information for N-fluorocinchona alkaloids, J. Am. Chem. Soc. 123 (2001) 7001-7009. |
| [31] | D.W. Fu, W. Zhang, H.L. Cai, et al., Supramolecular bola-like ferroelectric: 4-methoxyanilinium tetrafluoroborate-18-crown-6, J. Am. Chem. Soc. 133 (2011) 12780-12786. |








