b School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China
The aza-Michael addition is one of the most important organic reactions for C-N bond formation. This reaction not only plays an important role in the synthesis of biologically active natural products [1] but also serves as a tool to prepare synthetic precursors for pharmaceutical intermediates,β-amino alcohols and chiral auxiliaries [2]. It was usually catalyzed by various catalysts,such as polyaniline supported CuI,ultrasound,Ionic amino acids,etc. [3- 5]. However,there are still many deficiencies of these catalysts mentioned above,such as the requirement of long reaction time, harsh reaction conditions,tedious work-up and poor recyclability.
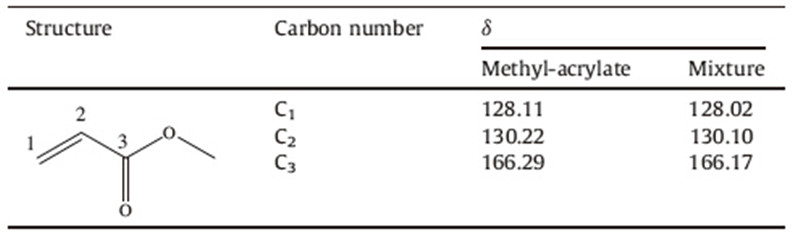
Owing to the interesting properties of ionic liquids (ILs),the application of ILs in organic synthesis has become an emerging area in organic chemistry. As solvents and/or catalysts [6, 7],they have been introduced in various organic transformations,such as Aldol reaction,Michael addition,borane reductions,Mannich reaction,Friedel-Crafts alkylation reaction,Knoevenagel condensation [8, 9, 10, 11]. There have been cases where imidazolium ILs were exploited to promote the aza-Michael reaction with various degree of success [12]. Very recently,four basic ionic liquids with dual catalytic roles based on DABCO and 3-chloro-1,2-propanediol were synthesized by our group (Fig. 1) and were successfully applied as catalysts in Knoevenagel condensation and aza-Michael addition reactions of α,β-unsaturated amides [13, 14]. Encouraged by the exciting results,we attempted to synthesize the task-specific ionic liquids to catalyze the aza-Michael addition reactions of secondary amines with α,β-unsaturated compounds (Scheme 1).
|
Download:
|
| Fig. 1.Structures of the [DABCO-PDO][X] ionic liquids. | |

|
Download:
|
| Scheme 1.Aza-Michael addition reaction of secondary amines and α,β-unsaturated compounds. | |
1H NMR and 13C NMR were recorded on a Bruker Avance DPX 400 spectrometer at 400 MHz and 100 MHz, respectively. Chemical shifts were reported in parts per million (δ),relative to the internal standard of tetramethylsilane (TMS). All the materials in reactions are commercially available and were used without further purification.
2.2. Preparation of the IL catalysts 2.2.1. Preparation of the IL [DABCO-PDO][X]To a magnetically stirred solution of DABCO (5.6 g,50 mmol) in anhydrous ethanol (50 mL) was added 3-chloro-1,2-propanediol (4.2 mL,50 mmol) and the mixture was heated at 80℃ for 24 h. The mixture was concentrated through rotatory evaporation under reduced pressure and the intermediate was obtained without any purification. Then the ion exchange reagents (KOAc/NaBF4/KPF6/ CF3SO3K) (0.02 mol) were added to a solution of the intermediate (0.02 mol) in anhydrous methanol (20 mL). The mixture was refluxed for 8 h and cooled for 0.5 h. The solids were filtered off and the liquid was evaporated under reduced pressure to give the corresponding IL catalyst.
2.2.2. Preparation of the IL [C3 DABCO][OAc]To a magnetically stirred solution of DABCO (0.56 g,5 mmol) in anhydrous ethanol (20 mL) was added 1-bromopropane (0.46 mL, 5 mmol) and the mixture was heated at 25℃ for 6 h. The mixture was concentrated through rotatory evaporation under reduced pressure and the intermediate was obtained without any purification. Then the ion exchange reagent KOAc (0.2 mol) was added to a solution of the intermediate (0.2 mol) in anhydrous methanol (15 mL). The mixture was refluxed for 4 h and cooled for 0.5 h. The solids were filtered off and the liquid was evaporated under reduced pressure to give the IL [C3DABCO][OAc] catalyst.
2.3. General procedure for the aza-Michael addition catalyzed by DABCO derived ILsA mixture of secondary amines (5.0 mmol),α,β-unsaturated compounds (5.0 mmol) and catalyst (15 mol%) was stirred at room temperature for an appropriate period of time and the progress of the reaction was monitored by TLC analysis. After the completion of the reaction,the reaction mixture was extracted with ethyl acetate. The ionic liquid could be further reused six times without further purification. The resulting residue (crude product) was purified by flash column chromatography (EtOAc). 3. Results and discussion
The DABCO based ionic liquids were synthesized by anion exchange after the reaction of 3-chloro-1,2-propanediol and DABCO (Scheme 2). And the specific synthetic method of [DABCO-PDO][X] is described in Section 2.
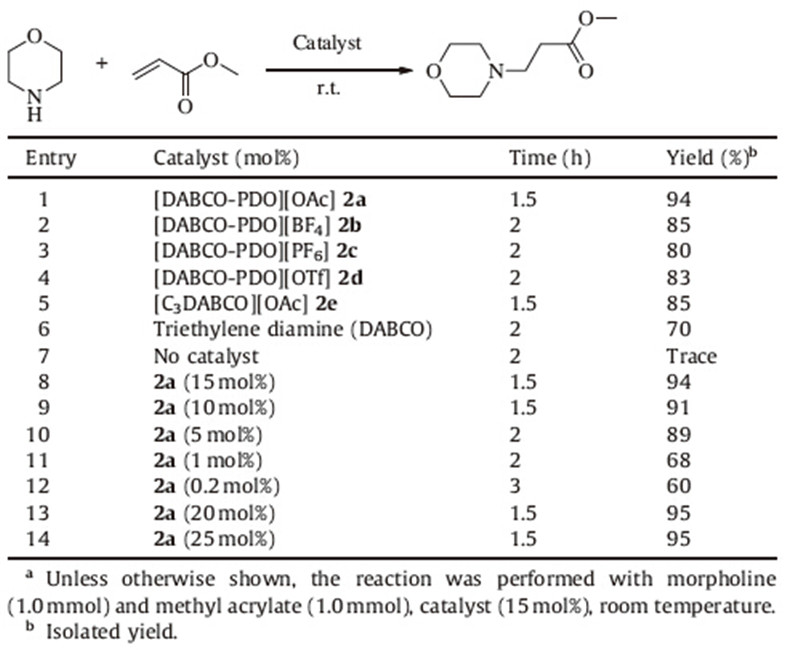
To evaluate the catalytic activity of the four different basic ILs in the aza-Michael addition reactions,the addition of morpholine and methyl acrylate was selected as a model reaction. The effects of catalyst loading and the nature of the catalysts were investigated in detail and the results are summarized in Table 1. As can be seen in Table 1,the addition reaction of morpholine and methyl acrylate proceeded smoothly in the presence of one of the catalysts 2a-2d ([DABCO-PDO][X]) and gave the desired product in good to excellent yields within 2.5 h (Table 1,entries 1-4). The ionic liquid that involves the acetate anion showed higher catalytic activity than those containing tetrafluoroborate, hexafluorophosphate or methylsulfonic anion. For comparison,we used triethylene diamine (DABCO) as the catalyst,the reaction time needed to be prolonged to 4 h albeit with the satisfied product (Table 1,entry 5). When the catalyst was not used,only trace amount of product was detected even after 10 h of reaction (Table 1,entry 6). [DABCOPDO][ OAc] (2a) was chosen as the optimal catalyst for further studies. To test the effect of catalyst loading on aza-Michael addition,we experimented with the model reaction under various catalyst loadings. The yield was observed to decrease with decreased catalyst loading in the range of 15-5 mol%,but the yields were still very high (Table 1,entries 7-9). When the amount of catalyst was further reduced from 5 mol% to 1 mol% and 0.2 mol%,the yields of the desired product significantly decreased even after long reaction time (Table 1,entries 10-11). When the amount of IL was increased from 15 mol% to 20 mol% and 25 mol%, neither the reaction time nor the yield was improved (Table 1, entries 12-13). These experiments show that the reasonable amount of the catalyst 2a is 15 mol%.
|
Download:
|
| Scheme 2.Synthesis of the [DABCO-PDO][X] ionic liquids. | |
| Table 1 Effect of catalyst on the aza-Michael addition of morpholine and methyl acrylate.a |
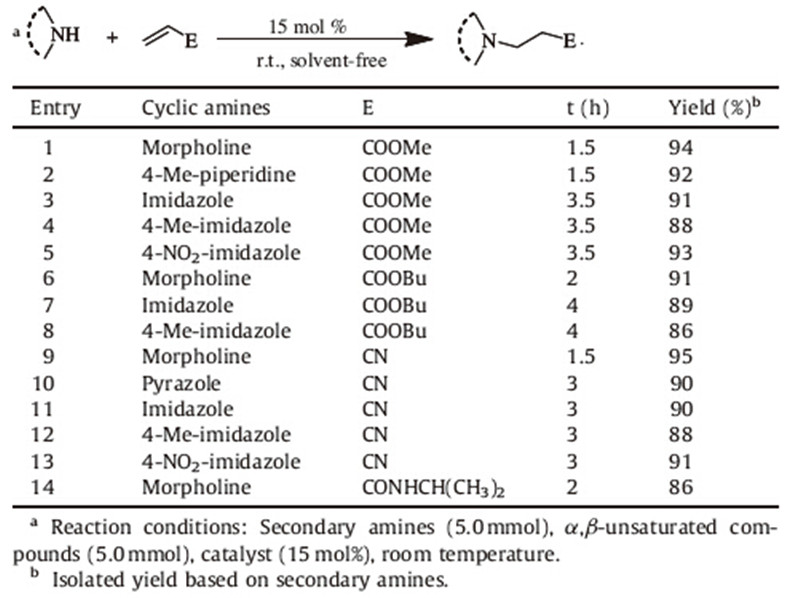
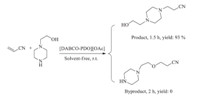
With the optimized reaction conditions in hand,we then examined the scope and limitations of this strategy for other structurally diverse substrates and the results are summarized in Table 2. All the reactions proceeded smoothly to afford good to excellent yields with 2a as a catalyst. There is a common reaction phenomenon that aliphatic amines reacted faster than imidazole, which has lower nucleophilic ability for its electron delocalization (Table 2,entries 1,2,6,9 vs. entries 3-5,7-8,10-13). Imidazoles with electron-withdrawing group gave much higher yields than those with electron-donating group (Table 2,entries 5,13 vs. entries 4,8,12). Moreover,the chain of acrylates can also influence the reaction process. The acrylates with the shorter chain (OMe) performed much better than that with large substituted group (OBu) (Table 2,entries 1 and 6). Meanwhile,acrylonitrile can react smoothly with secondary amines to give the corresponding products in 88%-95% yields (Table 2,entries 9-13). Finally, acrylamide is an efficient Michael acceptor,showing a broad substrate applicability of the DABCO-based catalysts (Table 2, entry 14). Finally,chemoselectivity of 2a was examined in the reaction of acrylonitrile and N-(2-hydroxyethyl)piperazine (Scheme 3). 93% Yield of adduct formed by nitrogen nucleophilic attack was obtained while a byproduct formed via oxygen nucleophilic addition was detected,demonstrating the excellent chemoselectivity of 2a.
| Table 2 Aza-Michael addition reaction of secondary amines and α,β-unsaturated compounds catalyzed by [DABCO-PDO][OAc]. |
|
Download:
|
| Scheme 3.Investigation of chem-selectivity of catalyst IT-DABCO@MNPs. | |
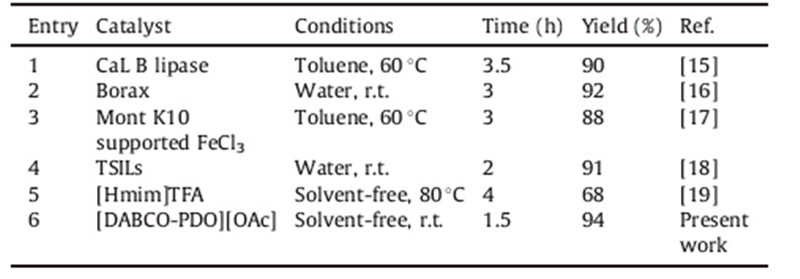
In order to show the merit of the present work,we compared the results of the reaction of methyl-3-morpholinopropanoate with those from published literature. As shown in Table 3,the IL [DABCO-PDO][OAc] can serve as an efficient catalyst with respect to reaction time and yield of the product.
| Table 3 Comparison results with different catalysts and conditions in the synthesis of methyl-3-morpholinopropanoate. |
A plausible mechanism for the reaction between morpholine and methyl acrylate catalyzed by [DABCO-PDO][OAc] is shown in Scheme 4. First,the hydrogen atom of morpholine can be captured by the nitrogen atom of the IL to form corresponding active nitrogen anion. Second,the hydrogen bonding interaction between the carbonyl group of the methyl acrylate and the hydroxyl groups of the [DABCO-PDO][OAc] decreases the electron density of carboxylic carbon atom and then activates the vinyl carbon. Thus, the active nitrogen anion of Michael donors attacks it much readily. The dual activation of aliphatic amines as well as α,β-unsaturated ingredients in the presence of catalyst [DABCO-PDO][OAc] allowed the reaction to proceed smoothly. This plausible mechanism for the aza-Michael addition catalyzed by [DABCO-PDO][OAc] has been proven by the test shown in Table 1. Without the two hydroxyl groups,the IL [C3DABCO][OAc] (entry 5,Table 1) shows a poorer catalytic ability than the [DABCO-PDO][OAc] (entry 1, Table 1). Moreover,for this reaction,the yield is strongly influenced by the basicity of the catalyst. Judging from the acidity order of their conjugate acid (HPF6 > CF3SO3H > HBF4 > CF3- F3COOH > CH3COOH) [20],the catalyst [DABCO-PDO][OAc] possesses the highest alkalinity among 2a-2d,which confirms the highest catalytic activity of 2a among the catalysts in Table 1.
|
Download:
|
| Scheme 4.A plausible mechanism for the reaction between morpholine and methyl acrylate catalyzed by [DABCO-PDO][OAc]. | |
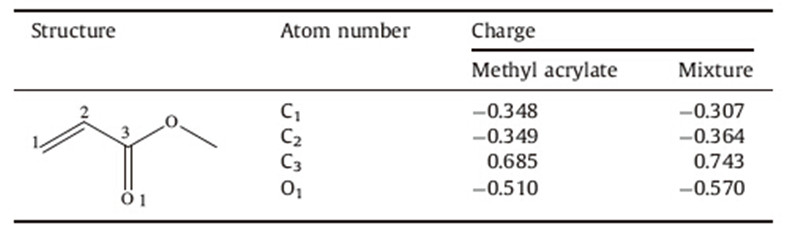
To support this plausible mechanism for the reaction between morpholine and methyl acrylate catalyzed by [DABCO-PDO][OAc], the charge of atom on methyl acrylate was calculated by Gaussian 03. The B3LYP/3-21G-optimized geometry of methyl acrylate provided the charge of -0.343 for C1 while the B3LYP/3-21Goptimized geometry of methyl acrylate-[DABCO-PDO][OAc] provided the charge of -0.307 for C1 (Table 4). A decrease in charge of 0.036 at C1 led to an increase of the electrophilicity of the methyl acrylate and the result suggests that the hydrogen bond between the hydroxyl groups of the IL and the carbonyl group of the methyl acrylate has been formed (Fig. S1 in Supporting information).
| Table 4 The charge of methyl acrylate and methylacrylate-[DABCO-PDO][OAc] mixture. |
This plausible mechanism could be further proven by comparison of 13C NMR spectra of methyl acrylate and the mixture of methyl acrylate and [DABCO-PDO][OAc]. As shown in Table 5,the chemical shift of carbonyl in methyl acrylate is 166.29 while it is 166.17 in mixture. The chemical shift offset by 0.12,which indicates the interaction between the hydroxyl groups of [DABCOPDO][ OAc] and the carbonyl group of the methyl acrylate [21].
| Table 5 13C NMR data of methyl acrylate and its corresponding mixture with [DABCOPDO][ OAc]. |
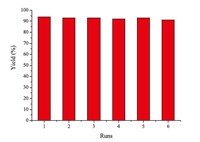
In order to demonstrate the industrial applicability of this methodology,the solvent-free aza-Michael addition of piperidine and methyl acrylate was carried out. A mixture of piperidine (5 mmol),methyl acrylate (5 mmol) and catalyst (0.75 mmol) was stirred at room temperature for an appropriate period of time. Once the reaction process was completed,ethyl acetate was added to extract the crude product,which was further purified by silica column chromatography to give the desired product. The recovered ionic liquid was reused directly in the subsequent reactions. As demonstrated in Fig. 2,the results show that the catalyst has no substantial loss in activity even after the six reuses. All reactions were completed within 1.5 h and gave excellent yields.
|
Download:
|
| Fig. 2.Reusability of the [DABCO-PDO][OAc]. | |
In conclusion,we have developed a mild and efficient method using DABCO-based ionic liquids as an efficient and recyclable catalyst for aza-Michael addition reactions of various secondary amines with α,β-unsaturated compounds at room temperature under solvent-free conditions. The reactions proceed smoothly and the desired products were obtained in good to excellent yields. The proposed mechanism was presented and verified by DFT calculation and carbon nuclear magnetic resonance experiment,respectively. The catalyst [DABCO-PDO][OAc] can be recycled and reused six times without noticeable loss of activity.
AcknowledgmentsWe are grateful for the financial supports for this research by the National Natural Science Foundation of China (Nos. 21106090 and 21176170),Foundation of Low Carbon Fatty Amine Engineering Research Center of Zhejiang Province (No. 2012E10033),and Zhejiang Provincial Natural Science Foundation of China (No. LY12B02004).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.11.018.
| [1] | L.W. Xu, C.G. Xia, X.X. Hu, An efficient and inexpensive catalyst system for the aza-Michael reactions of enones with carbamates, Chem. Commun. 20 (2003) 2570-2571. |
| [2] | V. Kapras, R. Pohl, I. Císařová, U. Jahn, Asymmetric domino aza-Michael addition/[3+2] cycloaddition reactions as a versatile approach to α, β, γ-triamino acid derivatives, Org. Lett. 16 (2014) 1088-1091. |
| [3] | M.L. Kantama, M. Roy, S. Roy, B. Sreedhar, R.L. De, Polyaniline supported CuI: an efficient catalyst for C-N bond formation by N-arylation of N(H)-heterocycles and benzyl amines with aryl halides and arylboronic acids, and aza-Michael reactions of amines with activated alkenes, Catal. Commun. 9 (2008) 2226-2230. |
| [4] | D. Bandyopadhyay, S. Mukherjee, L.C. Turrubiartes, B.K. Banik, Ultrasoundassisted aza-Michael reaction in water: a green procedure, Ultrason. Sonochem. 19 (2012) 969-973. |
| [5] | N. Morimoto, Y. Takeuchi, Y. Nishina, Ionic amino acids: application as organocatalysts in the aza-Michael reaction, J. Mol. Catal. A: Chem. 368 (2013) 31-37. |
| [6] | T.K.T. Truong, G. Vo-Thanh, Synthesis of functionalized chiral ammonium, imidazolium, and pyridinium-based ionic liquids derived from (-)-ephedrine using solvent-free microwave activation. Applications for the asymmetric Michael addition, Tetrahedron 66 (2010) 5277-5282. |
| [7] | Y.Dai, B.D.Li,H.D.Quan, C.X.Lü, [Hmim]3PW12O40: ahigh-efficientandgreencatalyst for the acetalization of carbonyl compounds, Chin. Chem. Lett. 21 (2010) 678-681. |
| [8] | D.E. Siyutkin, A.S. Kucherenko, S.G. Zlotin, A new (S)-prolinamide modified by an ionic liquid moiety—a high performance recoverable catalyst for asymmetric aldol reactions in aqueous media, Tetrahedron 66 (2010) 513-518. |
| [9] | A.G. Ying, L. Liu, G.F. Wu, et al., Aza-Michael addition of aliphatic or aromatic amines to a, b-unsaturated compounds catalyzed by a DBU-derived ionic liquid under solvent-free conditions, Tetrahedron Lett. 50 (2009) 1653-1657. |
| [10] | S. Sahler, H. Konnerth, N. Knoblauch, M.H.G. Prechtl, Hydrogen storage in amine boranes: ionic liquid supported thermal dehydrogenation of ethylene diamine bisborane, Int. J. Hydrogen Energy 38 (2013) 3283-3290. |
| [11] | X. Zheng, Y.B. Qian, Y.M. Wang, 2-Pyrrolidinecarboxylic acid ionic liquid as a highly efficient organocatalyst for the asymmetric one-pot Mannich reaction, Eur. J. Org. Chem. 2010 (2010) 515-522. |
| [12] | E.V. Matveeva, P.V. Petrovskii, Z.S. Klemenkova, N.A. Bondarenko, I.L. Odinets, A practical and efficient green synthesis of β-aminophosphoryl compounds via the aza-Michael reaction in water, Comptes Rendus Chimie 13 (2010) 964-970. |
| [13] | A.G. Ying, Y.X. Ni, S.L. Xu, et al., Novel DABCO based ionic liquids: green and efficient catalysts with dual catalytic roles for aqueous Knoevenagel condensation, Ind. Eng. Chem. Res. 53 (2014) 5678–5682. |
| [14] | A.G. Ying, Z.F. Li, J.G. Yang, et al., DABCO based ionic liquids: recyclable catalysts for aza-Michael addition of alpha, beta-unsaturated amides under solvent-free conditions, J. Org. Chem. 79 (2014) 6510–6516. |
| [15] | K.P. Dhake, P.J. Tambade, R.S. Singhal, B.M. Bhanage, Promiscuous Candida Antarctica lipase B-catalyzed synthesis of β-amino esters via aza-Michael addition of amines to acrylates, Tetrahedron Lett. 51 (2010) 4455–4458. |
| [16] | S. Hussain, S.K. Bharadwaj, M.K. Chaudhuri, H. Kalita, Borax as an efficient metalfree catalyst for hetero-Michael reactions in an aqueous medium, Eur. J. Org. Chem. 2007 (2007) 374–378. |
| [17] | V.R. Choudhary, D.K. Dumbre, S.K. Patil, FeCl3/Montmorillonite K10 as an efficient catalyst for solvent-free aza-Michael reaction between amine and a, b-unsaturated compounds, RSC Adv. 2 (2012) 7061–7065. |
| [18] | X. Liu, M. Lu, G. Gu, T. Lu, Aza-Michael reactions in water using functionalizedionic liquids as the recyclable catalysts, J. Iran. Chem. Soc. 8 (2011) 775–781. |
| [19] | M. Dabiri, P. Salehi, M. Bahramnejad, M. Baghbanzadeh, Ecofriendly and efficient procedure for hetero-Michael addition reactions with an acidic ionic liquid as catalyst and reaction medium, Monatsh Chem. 143 (2012) 109–112. |
| [20] | S. Mori, M. Ue, K. Ida, Electrolyte for aluminum electrolytic capacitor, US Patent US4774011 (1988). |
| [21] | H. Guo, J.L. Wang, X. Li, D.S. Lü, X.F. Lin, Oxa-Michael addition catalyzed by amidebased acidic ionic liquids, Chin. J. Catal. 32 (2011) 162–165. |