b School of Chemical and Engineering, Shanghai Institute of Technology, Shanghai 201418, China
Boron chemistry is one of the most important subjects in organic chemistry,and it attracted increasing attention because of their intrinsic scientific importance and industrial applications. Much attention has been focused on looking for easy and efficient ways to prepare organoboron reagents,which is an important reagent family in organic synthesis [1]. The traditional C-B formation via addition reaction of borane with alkenes or alkynes was conducted under harsh conditions. More recently,the C-B cross-coupling reaction of alkyl halides showed many advantages [2]. Due to the low price and toxicity,Cu salts have been widely reported as catalysts for cross-coupling reactions,which are valuable reactions for synthetic organic chemistry [3]. Coppercatalyzed reaction showed advantages including low toxicity, mild reaction conditions and wide substrate tolerance,but may suffer from several limitations such as low conversion efficiency [4]. Nonetheless,Marder and Liu et al. recently reported that CuI in the presence of phosphines catalyzed the borylation of primary and secondary alkyl halides and pseudo halides [5]. Previously,we reported that amino acid ligands could promote several types of transition metal-catalyzed reactions [6]. The amino acids as ligands are especially interesting,because they are inexpensive, conveniently available,environmentally benign,and structurally diverse. Therefore,we recently initiated a project to study the copper-catalyzed borylation reactions with amino acids as ligands. Herein,we disclosed a method for mild copper-catalyzed borylation using amino acids as ligands,which showed low cost, low toxicity and high conversion efficiency. 2. Experimental
General procedure of amino acid/Cu borylation reaction: a mixture of bis(pinacolato)diboron (B2pin2,1a) (0.25 mmol),alkyl halide (0.38 mmol),Cu salt (10 mol% forB2pin2) and ligand (13 mol% for B2pin2),in 0.5 mLsolvent is reacted under argon at 25 ℃ for 18 h. Then the solvent is evaporated,the residue is purified by chromatography to afford pure product. 3. Results and discussion
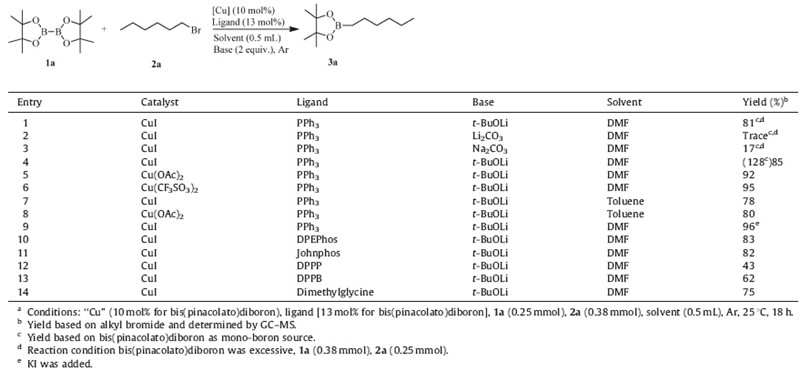
Initially we repeated the previous procedure to couple diboron reagent of B2pin2 with alkyl electrophile (Table 1,entry 1),and comparable yield (81%) was obtained. After a careful examination of the crude extract of the reaction mixture,we found excessive B2pin2 were consumed. Therefore,we changed to use excessive bromohexane (0.38 mmol) in this reaction. A significantly high yield (128%) was observed,as calculated on the basis of B2pin2 as mono-boron source (entry 2). The yield in excess of 100% indicated that the by products of B2pin2 should be involved in the reaction. So,we calculated the yield based on alkyl bromide,and obtained the 85% yield,meaning much higher efficient boron conversion.
| Table 1 Optimization studies for ‘‘Cu’’ catalyze the reaction of diboron reagent (1a) with alkyl electrophile (1b).a |
To develop more effective catalyst systems for this reaction, the effects of various copper salts,bases,solvents and ligands were investigated. The detailed results were listed in Table 1. Marder et al. [7] have demonstrated that t-BuOK is an effective generator of active nucleophile. When we replaced t-BuOLi with other bases,such as Li2CO3 and Na2CO3,the yields decreased dramatically. To explore the role of t-BuOLi in the reaction,the verification experiments were carried out under the same conditions without bromohexane. B2pin2 were completely degraded in the presence of t-BuOLi,indicating that t-BuOLi is involved in the initial step.
Fixing t-BuOLi as base,we explored the reaction with various Cu salts. Cu(CF3SO3)2 gave a better yield (95%) than CuI (85%) and Cu(OAc)2 (92%) (entries 5 and 6). The reactions in DMF were better than in toluene,maybe due to the solubility of salts (entries 7 and 8). With the addition of KI,the yield was as high as 96%. The various ligands were performed in the presence of CuI/t-BuOLi system in DMF,and it was found that ligands played an important role in the reactions. Phosphine ligands of DPEPhos,Johnphos gave much better yields (entries 10 and 11) than the DPPP and DPPB (entries 12 and 13). Notably,the amino acid of dimethylglycine acted as a good ligand as well,and yield was as high as 75%.
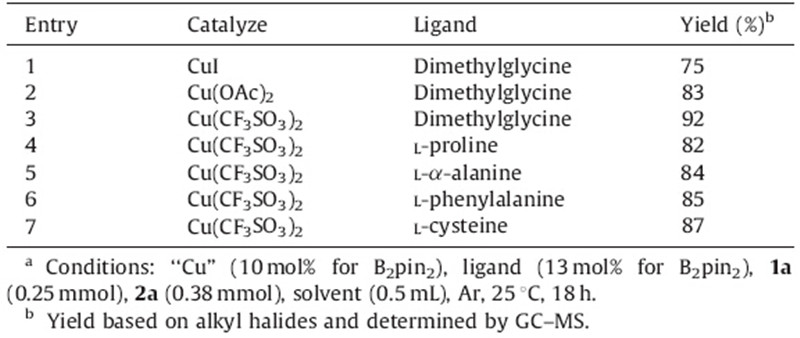
The application of amino acids and their derivatives as ligands in synthetic organic chemistry promoted by the ruthenium, rhodium or iridium complexes has attracted more and more attentions [8]. Therefore,we turned our attention to amino acid catalyzed borylation of B2pin2 with bromohexane (Table 2). Cu(CF3SO3)2 was better Cu salt than Cu(OAc)2 and CuI,and gave the yield as high as 92%. Using the reaction procedure for Cu(CF3SO3)2/dimethylglycine,we also examined other amino acids as ligands for the borylation. It is found that in addition to dimethylglycine,one can also use natural amino acids of L-proline, L-α-alanine,L-phenylalanine and L-cystine. They all give quite similar yields regardless of the properties of the side chains. It is worthy to mention that the coupling between bromohexane or B2pin2 with the amino groups on the amino acid moiety was negligible in all the amino acid catalyzed borylation reaction as tested by GC/MS. Therefore,we conclude the reaction conditions in entry 3,Table 2 were optimal for further investigation on the reaction scope.
| Table 2 The reaction of diboron reagent (1a) with alkyl electrophile (1b) with various amino acids as ligands.a |
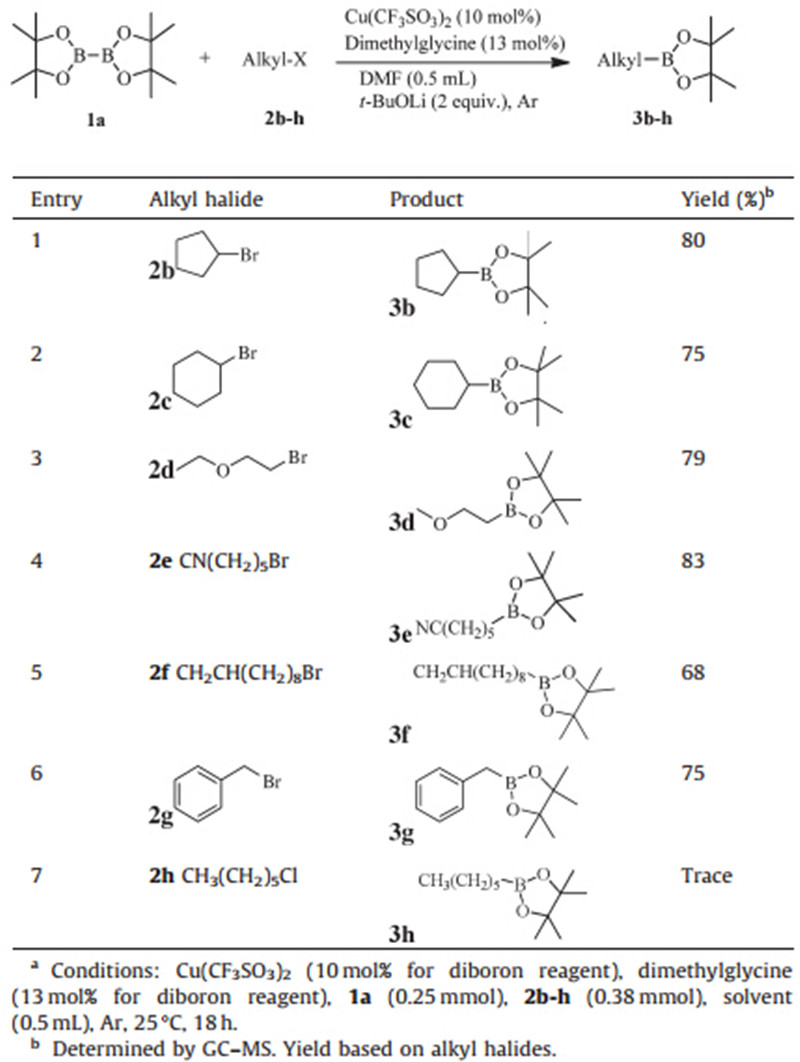
With the conditions in entry 3 (Table 2),we set out to carry out the reactions of B2pin2 with various alkyl bromide substrates,as shown in Table 3. The secondary alkyl bromides of cyclopentane and cyclohexane bromides worked fairly well,giving the desired products in good yields of 80% and 75%,respectively (entries 1 and 2). For primary alkyl bromides,it was found that a variety of substituent,such as ether,nitrile and alkene,could be tolerated in the amino acid-mediated amidation reaction. Interestingly,alkene group kept stable (entries 3-5). The reaction also proceeded well with aryl bromide (yield 75%). To our disappointment,alkyl chloride failed to give the corresponding product,due to the low activity of chloro (entry 7).
| Table 3 Cu-catalyzed borylation of various alkyl halides.a |
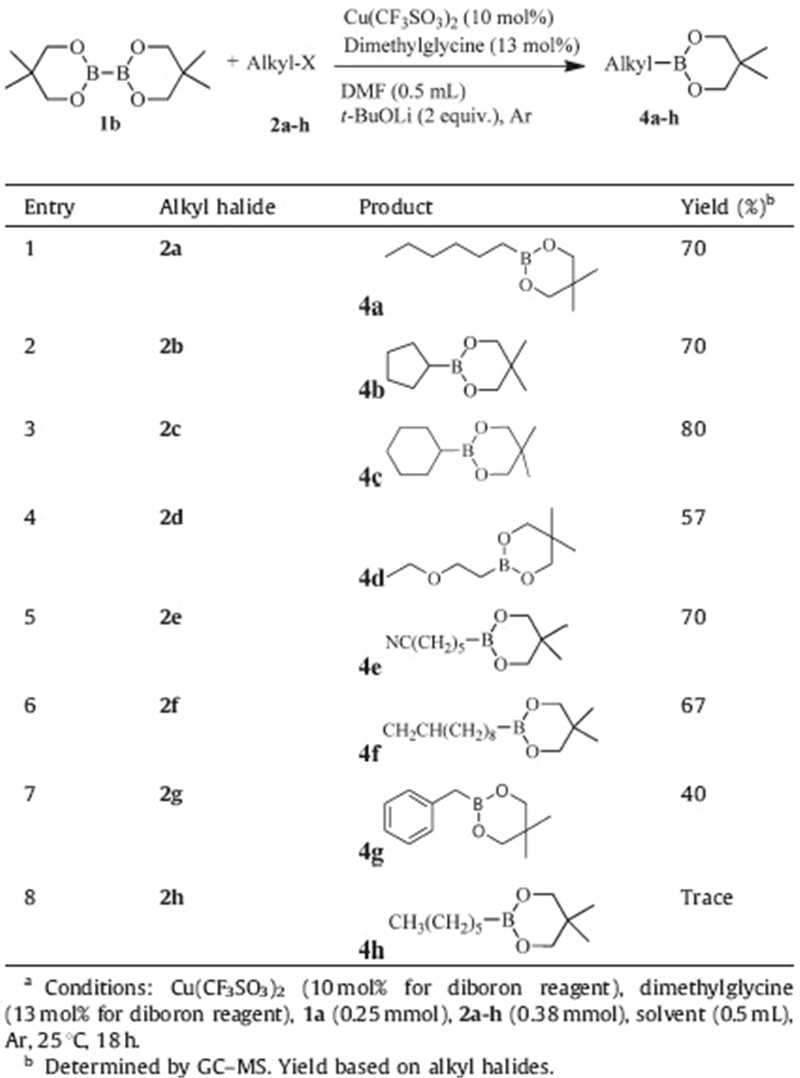
Furthermore,the reactions between bis(neopentyl glycolato)- diboron (1b) with alkyl bromides were also investigated under identical conditions (Table 4). To our delight,most substituted alkyl bromides were well tolerated under the optimized conditions, leadingto the desired products inmoderate to good yields. Similar as B2pin2,both of primary and secondary alkyl bromides reacted well (entries 1-3),and many substituents could be tolerated,including ether,nitrile,alkene and benzyl groups (entries 4-7). However,the alkyl chloride still was a problem (entry 8). It need to be noted that the aromatic diboron sources,such as bis(catecholato)diboron,also were tried in this condition. However,they failed to give the corresponding products.
| Table 4 Cu-catalyzed the reaction of diboron reagent (2a) with various alkyl halides.a |
Finally,the mechanism of the reaction was investigated. At present,the mechanism of the Cu(I) catalyzed borylation reaction is not completely clear yet. To the best of our knowledge, boryllithium can react with a variety of electrophiles [9]. Also,it has been reported that i-PrCuOR can catalyze the base-free β-boration of crotonaldehyde with B2pin2 [10]. To investigate the mechanism,we did some additional reactions. As mentioned before,Cu catalysts would greatly promote the decomposition of B2pin2,and the reaction of B2pin2 with t-BuOLi could not occur without Cu salts. We observed Bpin-O-t-Bu,but Bpin-Li was not confirmed,due to the pool stability. Therefore,a possible mechanism was presented as in Scheme 1.
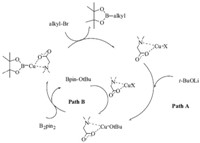
|
Download:
|
| Scheme 1.Possible mechanism for copper-catalyzed borylation of alkyl halides. | |
In summary,we have developed an environmental friendly and mild method for copper-catalyzed borylation of alkyl halides,using copper catalyst and amino acid ligands. The reactions proceeded smoothly in the presence of Cu(II) trifluoromethanesulfonate, dimethylglycine and t-BuOLi under mild conditions. Additionally, the reaction proceeds with high yields using diboronate compounds as bi-boron sources for borylation. Future work is focused on the exploration of the borylation of chloroalkanes.
Acknowledgments
We gratefully acknowledge financial support from the Eastern Scholar,Shanghai Pujiang Program,Key Subject of Shanghai Municipal Education Commission,Natural Science Foundation of Shanghai (No. 12ZR1410500),and NSFC (Nos. 21102088, 51202138 and 21174081).
| [1] | T.C. Atack, R.M. Lecker, A.P. Cook, Iron-catalyzed borylation of alkyl electrophiles, J. Am. Chem. Soc. 136 (2014) 9521-9523. |
| [2] | (a) P.J. Unsworth, D. Leonori, V.K. Aggarwal, Stereocontrolled synthesis of 1,5-stereogenic centers through three-carbon homologation of boronic esters, Angew. Chem. Int. Ed. 53 (2014) 9846-9850; (b) K.L. Billingsley, T.E. Barder, S.L. Buchwald, Palladium-catalyzed borylation of aryl chlorides: scope, applications, and computational studies, Angew. Chem. 119 (2007) 5455-5459; (c) M.A. Larsen, J.F. Hartwig, Iridium-catalyzed C-H borylation of heteroarenes: scope, regioselectivity, application to late-stage functionalization, and mechanism, J. Am. Chem. Soc. 136 (2014) 4287-4299; (d) C.W. Liskey, J.F. Hartwig, Iridium-catalyzed C-H borylation of cyclopropanes, J. Am. Chem. Soc. 135 (2013) 3375-3378; (e) L.S. Zhang, G.H. Chen, X. Wang, et al., Direct borylation of primary C-H bonds in functionalized molecules by palladium catalysis, Angew. Chem. 126 (2014) 3980-3984; (f) J. Yu, L. Zhang, G.B. Yan, Metal-free, visible light-induced borylation of aryldiazonium salts: a simple and green synthetic route to arylboronates, Adv. Synth. Catal. 354 (2012) 2625-2628; (g) C.T. Yang, Z.Q. Zhang, Y.C. Liu, L. Liu, Copper-catalyzed cross-coupling reaction of organoboron compounds with primary alkyl halides and pseudohalides, Angew. Chem. Int. Ed. 50 (2011) 3904-3907; (h) G.B. Yan, Y.B. Jiang, C.X. Kuang, et al., Nano-Fe2O3-catalyzed direct borylation of arenes, Chem. Commun. 46 (2010) 3170-3172. |
| [3] | For selected work on the copper-catalyzed reactions, see: (a) X. Zhang, H. Yi, Z.X. Liao, et al., Copper-catalysed direct radical alkenylation of alkyl bromides, Org. Biomol. Chem. 12 (2014) 6790-6793; (b) X.Y. Li, B.J. Li, J.S. You, J.B. Lan, Copper-catalysed oxidative C-H/N-H crosscoupling between formamides and amides through chelation-assisted N-H activation, Org. Biomol. Chem. 11 (2013) 1925-1928; (c) C.T. Yang, Z.Q. Zhang, J. Liang, et al., Copper-catalyzed cross-coupling of nonactivated secondary alkyl halides and tosylates with secondary alkyl Grignard reagents, J. Am. Chem. Soc. 134 (2012) 11124-11127. |
| [4] | N. Miyaura, A. Suzuki, Palladium-catalyzed cross-coupling reactions of organoboron compounds, Chem. Rev. 95 (1995) 2457-2483. |
| [5] | C.T. Yang, Z.Q. Zhang, H. Tajuddin, et al., Alkylboronic esters from copper-catalyzed borylation of primary and secondary alkyl halides and pseudohalides, Angew. Chem. Int. Ed. 51 (2012) 528-532. |
| [6] | (a) W. Deng, Y.F. Wang, Y. Zou, L. Liu, Q.X. Guo, Amino acid-mediated Goldberg reactions between amides andaryl iodides, Tetrahedron Lett.45 (2004) 2311-2315; (b) W. Deng, Y. Zou, Y.F. Wang, L. Liu, Q.X. Guo, CuI-catalyzed coupling reactions of aryl iodides and bromides with thiols promoted by amino acid ligands, Synlett (2004) 1254-1258; (c) W. Deng, L. Liu, C. Zhang, M. Liu, Q.X. Guo, Copper-catalyzed cross-coupling of sulfonamides with aryl iodides and bromides facilitated by amino acid ligands, Tetrahedron Lett. 46 (2005) 7295-7298; (d) X. Cui, Z. Li, C.Z. Tao, et al., N,N-dimethyl-b-alanine as an inexpensive and efficient ligand for palladium-catalyzed Heck reaction, Org. Lett. 8 (2006) 2467-2470; (e) X. Cui, J. Li, Z.P. Zhang, et al., Pd(quinoline-8-carboxylate)2 as a low-priced, phosphine-free catalyst for Heck and Suzuki reactions, J. Org. Chem. 72 (2007) 9342-9345. |
| [7] | C. Kleeberg, Z.Y. Lin, T.B. Marder, A facile route to aryl boronates: room-temperature, copper-catalyzed borylation of aryl halides with alkoxy diboron reagents, Angew. Chem. 121 (2009) 5454-5458. |
| [8] | For selected work on amino acids and their derivatives as ligands in synthetic organic chemistry, see: (a) D.W. Ma, Q. Cai, Copper/amino acid catalyzed cross-couplings of aryl and vinyl halides with nucleophiles, Acc. Chem. Res. 41 (2008) 1450-1460; (b) D.W. Ma, Q. Cai, H. Zhang, Mild method for Ullmann coupling reaction of amines and aryl halides, Org. Lett. 5 (2003) 2453-2455; (c) J.S. Zheng, H.N. Chang, F.L. Wang, L. Liu, Fmoc synthesis of peptide thioesters without post-chain-assembly manipulation, J. Am. Chem. Soc. 133 (2011) 11080-11083; (d) S. Gladiali, E. Alberico, Asymmetric transfer hydrogenation: chiral ligands and applications, Chem. Soc. Rev. 35 (2006) 226-236; (e) C.T. Yang, Y. Fu, Y.B. Huang, et al., Room-temperature copper-catalyzed carbon-nitrogen coupling of aryl iodides and bromides promoted by organic ionic bases, Angew. Chem. Int. Ed. 48 (2009) 7398-7401. |
| [9] | For selected work on boryllithium, see: (a) Y. Segawa, Y. Suzuki, M. Yamashita, K. Nozaki, Chemistry of boryllithium: synthesis, structure, and reactivity, J. Am. Chem. Soc. 130 (2008) 16069-16079; (b) P. Jaramillo, P. Pérez, P. Fuentealba, Chemical reactivity descriptors for ambiphilic reagents: dual descriptor, local hypersoftness, and electrostatic potentia, J. Phys. Chem. A 113 (2009) 6812-6817; (c) K. Nozaki, Y. Aramaki, M. Yamashita, S.H. Ueng, M.M. Curran, Boryltrihydroborate: synthesis, structure, and reactivity as a reductant in ionic, organometallic, and radical reactions, J. Am. Chem. Soc. 132 (2010) 11449-11451. |
| [10] | A. Boneta, V. Lilloa, J. Ramíreza, M.M. Requejob, E. Fernández, The selective catalytic formation of b-boryl aldehydes through a base-free approach, Org. Biomol. Chem. 7 (2009) 1533-1535. |