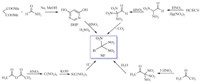
Nitroform (trinitromethane,NF),which has high oxygen content,is an important intermediate for the preparation of numerous high explosives and propellants. NF is involved in many reactions such as addition reaction [1],condensation reaction [2], substitution reaction [3]. When reacted with unsaturated compounds, NF afforded multi-nitroalkane derivatives,therefore it can be used for the synthesis of a system explosive containing nitroform group,such as 1-(2,2,2-trinitroethylamino)-2-nitroguanidines [4] and 1,1,1,3-tetranitro-3-azabutane [5]. Due to the acidic hydrogen atom,nitroform can generate a lot of nitroformate salt derivatives,for example hydrazinium nitroformate (HNF). HNF have many aspects of the application: it can be used as an oxidizer in,inter alia,rocket propellants and it is also chlorine-free,a desired property in modern propellant compositions [6, 7]. In the literature,the most well-known preparation method of nitroform is the nitration of acetylene with nitric acid,which using an expensive and toxic mercury nitrate as catalyst,this method is obvious drawbacks from an environmental point of view. Other preparation methods of nitroform include the nitration of some other suitable compounds,such as acetone or isopropanol, hydrolysis of tetranitromethane and the nitration-hydrolysis of pyrimidine-4,6-dione [8, 9] (Fig. 1) of acetone. Due to the volatility and flammability of acetone,the method with acetone involved in, is seriously dangerous; the method with tetranitromethane involved in,requires water vapor distillation process,and this make it difficult to prepare the raw materials.
|
Download:
|
| Fig. 1. Preparations of nitroform reported. | |
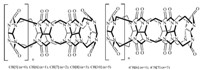
Cucurbi[6]turil (CB[6]) is a cyclic oligomer of 6 units of glycoluril linked by 12 methylene bridges [10]. A series of its homologues and derivatives have been reported from1981 [11] (Fig. 2),and this kind of molecules have a high degree of symmetry and rigid structures. Over the past decade,the particular properties of cucurbiturils, including the binding of positively charged molecules at the two carbonyl portals and of neutralmolecules in the hydrophobic cavity, are the focus of attention in supramolecular chemistry. They have been widely used in many fields,such as drug delivery [12], molecular machines [13],supramolecular polymers [14],sensing ensembles [15],and biomimetic systems [16].
|
Download:
|
| Fig. 2.The structures of cucurbituril homologues. | |
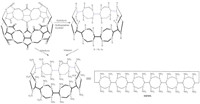
Thought of the carbonyl groups of CB[6] substituted by nitro groups,a special class of high energy density compound may be devised (Fig. 3),and we conceived a very wonderful name as crown explosive,abbreviated as HHMX,because this compound is a cyclic oligomer of 6 units of HMX linked by 6 symmetric methylene C-C bond bridges. It should be noted that,so far,the HHMX is single energetic molecule which contains the largest number of explosion groups (N-NO2) (up to 24,usually 6 such as HMX).
|
Download:
|
| Fig. 3.Design of HHMX. | |
When CB[6] was nitrolyzed in acetic anhydride with fuming nitric acid,nitroform instead of the expected HHMX was produced. Herein,we reported these results. 2. Experimental
All reagents (AR) and the distilled water were purchased from Beijing Chemical Reagent Co. Melting points were recorded on XT4 microscope melting point apparatus (uncorrected). Infrared (IR) spectra were recorded in Perkin Elmer FT-IR spectrophotometer with KBr pellets. 1H NMR spectra were registered on a Bruker spectrometer (400 Hz) with TMS as the internal standard. Mass spectra were recorded on ZAB-HS mass spectrometer using ESI ionization. 2.1. General procedure for the synthesis of CB[n]
CB[n] (n = 5-8) were prepared according to the literature [17].
CB[5]: 30 g,23.1% yield; mp > 300 ℃; 1H NMR (D2SO4/D2O, 400 MHz): δ 3.29 (d,12H,J = 16.0 Hz),2.61 (s,12H),1.35 (d,12H,J = 16.0 Hz); IR (KBr,cm-1): ν 3445,2999,2937,1739,1638,1477, 1417,1377,1328,1293,1233,1190,964,809,795,765,673.
CB[6]: 35 g,26.9% yield; mp > 300 ℃; 1H NMR (D2SO4/D2O, 400 MHz): δ 2.73 (d,10H,J = 16.0 Hz),3.17 (s,10H),1.90 (d,10H, J = 16.0 Hz); IR (KBr,cm-1): ν 3441,2998,2932,1735,1475,1416, 1376,1326,1295,1234,1190,1147,964,801,758,673.
CB[7]: 23 g,17.7% yield; mp > 300 ℃; 1H NMR (D2SO4/D2O, 400 MHz): δ 2.76 (d,14H,J = 16.0 Hz),2.61 (s,14H),1.32 (d,14H, J = 16.0 Hz); IR (KBr,cm-1): ν 3445,2999,2934,1732,1476,1420, 1376,1325,1293,1234,1191,967,825,806,759,674.
CB[8]: 10.15 g,7.8% yield; mp > 300 ℃; IR (KBr,cm-1): ν 3445, 3002,2925,1725,1474,1425,1375,1319,1293,1231,1191, 1157,993,970,830,808,759,675. 2.2. General procedure for the synthesis of sodium nitroformate
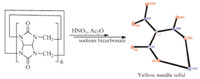
CB[n] (0.2 g) was added to a mixture of 5 mL of acetic anhydride and 5 mL of fuming nitric acid at room temperature,and stirred for 10 min. The mixture was heated slowly to 50 ℃ and kept it for 2 h, then cooled to room temperature. On dilution of the solution with 100 mL of iced water,there are some solid generated. The resulting precipitate was filtered off and the filtrate was extracted with ethyl acetate (3×20 mL). The extracted solution was washed with dilute sodium bicarbonate solution and dried by anhydrous sodium sulfate. The dried organic phase was evaporated in air to give bright yellow solid,it is sodium nitroformate.
IR (KBr,cm-1): ν 1534,1483,1413,1280,1173,792,737; MS (ESI) m/z (%): 150 (M-Na+),104 (M-Na+-NO2),45.9 (NO2). X-ray crystal data for NaC(NO2)3. CN3NaO6,Mr = 173.03,tetragonal, P41212 (no. 92),a = 7.1889(5),b = 7.1889(5),c = 9.8258(7) Å,α = 90°,β = 90°,γ = 90°,V = 507.79(6) Å3,Z = 4,ρcalcd = 2.263 g cm-3,F(0 0 0) = 344.0,T = 100.3 K. The CCDC ref. numbers is 1017954. 3. Results and discussion
In most cases,cucurbituril[6] was destroyed,and a yellowish solid was collected when CB[6] was nitrolyzed in acetic anhydride- nitric acid mixtures. The structure of the product was characterized as sodium nitroformate by spectral data. For example,its IR spectrum showed the strong absorption peak at 1280,1488 and 1534 cm-1 for nitro group. The mass spectrum given the molecular ion peak at m/z 150,and daughter ion peak at m/z 104 (M-NO2). So the product was sodium nitroformate (NaNF) and which was confirmed undoubtedly by its X-ray determination (Fig. 4).
|
Download:
|
| Fig. 4.Nitrolysis of CB[6]. | |
There are many nitroformate salts such as potassium nitroformate, ammonium nitroformate,silver nitroformate and hydrazinium nitroformate in the literature [8],but none of NaNF has been reported prior to this work. Sodium nitroformate is not very stable under common conditions,it is easily decomposed when both the temperature and the humidity are relatively higher. We have done a preliminary study on the solubility of sodium nitroformate,and found that it was relatively easy to dissolve in tetrahydrofuran,methanol,water,ethyl acetate,acetonitrile, acetone,ethanol,slightly soluble in diethyl ether,not soluble in petroleum ether,dichloromethane,1,2-dichloroethane,chloroform, benzene,toluene,cyclohexane and hexane.
Similarly,sodium nitroformate could be obtained by the nitrolysis of CB[6] homologues such as CB[n] (n = 5,7,8) under the same condition (Fig. 5). Moreover,the cucurbituril mixture, directly produced by the condensation of glycoluril and formaldehyde without isolation,can be nitrolyzed to produce NaNF.
It is stressed that this method for the synthesis of nitroform is a convenient method,because of the stability of cucurbituril and the mild condition. So this procedure provides a lower risk,inexpensive, novel preparation. 4. Conclusion
In this study,CB[n] (n = 5-8) and their mixture were nitrolyzed with fuming nitric acid in acetic anhydride to produce nitroform. This process,with mild reaction conditions,provides a lower risk, inexpensive,novel preparation of nitroform.
Acknowledgment
This work was supported by the grant of Beijing Institute of Technology.
| [1] | H. Shechter, H. Cates Jr., Addition reactions of trinitromethane and a,b-unsaturated ethers, J. Org. Chem. 26 (1961) 51-53. |
| [2] | H. Feuer, W.A. Swarts, Chemistry of trinitromethane. IV. Preparation of N-nitro-Ntrinitroethylamino alcohols, J. Org. Chem. 27 (1962) 1455-1457. |
| [3] | I.V. Ovchinnikov, A.S. Kulikov, M.A. Epishina, N.N. Makhova, V.A. Tartakovsky, Synthesis of N-trinitroethyl derivatives of linear and heterocyclic nitrogen-containing compounds, Russ. Chem. Bull. Int. Ed. 54 (2005) 1346-1349. |
| [4] | E.L. Metelkina, T.A. Novikova, 2-Nitroguanidine derivatives. Synthesis and structure of 1-(2,2,2-trinitroethylamino)-and 1-(2,2-dinitroethylamino)-2-nitroguanidines, Russ. J. Org. Chem. 38 (2002) 1378-1379. |
| [5] | T.M. Klapötke, B. Krumm, M. Scherr, G. Spieß, F.X. Steemann, Facile synthesis and crystal structure of 1,1,1,3-tetranitro-3-azabutane, Z. Anorg. Allg. Chem. 8 (2008) l244-l1246. |
| [6] | H.F.R. Schöer, W.H.M. Welland-Veltmans, J. Louwers, et al., Overview of the development of hydrazinium nitroformate, J. Propuls. Power 18 (2000) 131-137. |
| [7] | H.F.R. Schöer, W.H.M. Welland-Veltmans, J. Louwers, et al., Overview of the development of hydrazinium nitroformate-based propellants, J. Propuls. Power 18 (2002) 138-145. |
| [8] | (a) M. Göbel, T.M. Klapötke, P.Z. Mayer, Crystal structures of the potassium and silver salts of nitroform, Anorg. Allg. Chem. 632 (2006) 1043-1050; (b) M. Göbel, T.M. Klapötke, Potassium-, ammonium-, hydrazinium-, guanidinium-, aminoguanidinium-, diaminoguanidinium-, triaminoguanidinium-and melaminiumnitroformate-synthesis, characterization and energetic properties, Anorg. Allg. Chem. 633 (2007) 1006-1017; (c) J. Zhang, T.L. Zhang, J.G. Zhang, et al., Synthesis, crystal structure and thermal decomposition character of [Zn(CHZ)3][C(NO2)3]2·(H2O)2 (CHZ = carbohydrazide), Struct. Chem. 19 (2008) 321-328; (d) Y.G. Huang, H.X. Gao, B. Twamley, J.M. Shreeve, Synthesis and characterization of new energetic nitroformate salts, Eur. J. Inorg. Chem. 14 (2007) 2025-2030. |
| [9] | (a) P. Liang, Tetranitromethane, Org. Synth. 21 (1941) 105-107; (b) D.E. Welch, Process for Producing Nitroform, US 3491160, 1970; (c) A. Langlet, V.L. Nikolaj, U. Wellmar, P. Goede, Formation of nitroform in the nitration of gem-dinitro compounds, Propellants Explos. Pyrotech. 29 (2004) 344-348. |
| [10] | R. Behrend, E. Meyer, F. Rusche, Ueber condensationsproducte aus glycoluril und formaldehyd, Liebigs Ann. Chem. 339 (1905) 1-37. |
| [11] | (a) J. Kim, I.S. Jung, K. Kim, et al., New cucurbituril homologues: syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril (n = 5, 7, and 8), J. Am. Chem. Soc. 122 (2000) 540-541; (b) A.I. Day, A.P. Arnold, R.J. Blanch, B. Snushall, Controlling factors in the synthesis of cucurbituril and its homologues, J. Org. Chem. 66 (2001) 8094-8100; (c) A.I. Day, R.J. Blanch, A.P. Arnold, et al., A cucurbituril-based gyroscane: a new supramolecular form, Angew. Chem. Int. Ed. 41 (2002) 275-277; (d) S. Liu, P.Y. Zavalij, L. Isaacs, Cucurbit[10]uril, J. Am. Chem. Soc. 127 (2005) 16798-16799; (e) X.J. Cheng, L.L. Liang, Z. Tao, et al., Twisted cucurbit[14]uril, Angew. Chem. Int. Ed. 52 (2013) 7252-7255; (f) L. Isaacs, S.K. Park, S. Liu, et al., The inverted cucurbit[n]uril family, J. Am. Chem. Soc. 127 (2005) 18000-18001. |
| [12] | V.D. Uzunova, C. Cullinane, K. Brix, W.M. Nau, A.I. Day, Toxicity of cucurbit[7]uril and cucurbit[8]uril: an exploratory in vitro and in vivo study, Org. Biomol. Chem. 9 (2010) 2037-2042. |
| [13] | Y.H. Ko, E. Kim, I. Hwang, K. Kim, Supramolecular assemblies built with host-stabilized charge-transfer interactions, Chem. Commun. 13 (2007) 1305-1315. |
| [14] | (a) E.A. Appel, F. Biedermann, O.A. Scherman, et al., Supramolecular cross-linked networks via host-guest complexation with cucurbit[8]uril, J. Am. Chem. Soc. 132 (2010) 14251-14260; (b) F. Sakai, Z.W. Ji, J.H. Liu, G.S. Chen, M. Jiang, A novel supramolecular graft copolymer via cucurbit[8]uril-based complexation and its self-assembly, Chin. Chem. Lett. 24 (2013) 568-572; (c) H. Chen, H. Yang, W.C. Xu, W.B. Tan, A fluorescent guest used to determinate the effective content of CB[8] and to further detect methyl viologen, Chin. Chem. Lett. 24 (2013) 857-860. |
| [15] | G. Ghale, V. Ramalingam, A.R. Urbach, W.M. Nau, Determining protease substrate selectivity and inhibition by label-free supramolecular tandem enzyme assays, J. Am. Chem. Soc. 19 (2011) 7528-7535. |
| [16] | J.M. Chinai, A.B. Taylor, L.M. Ryno, et al., Molecular recognition of insulin by a synthetic receptor, J. Am. Chem. Soc. 23 (2011) 8810-8813. |
| [17] | (a) F. Meng, Modified Cucurbituril-Cyclopentyl Substituted Cucurbituril Synthesis and Separation, M.S. thesis, Guizhou University, 2006; (b) Q. Bi, Y.P. Hu, Q. Yang, C.L. Ma, D.L. Li, A two-step approach for cucurbit[n]uril compound separating by water and hydrochloric acid, Chin. J. Org. Chem. 27 (2007) 880-884. |





