At the beginning of the 20th century,Mario Betti discovered the three-component reaction of 2-naphthol,aryl aldehydes and ammonia or amines for the synthesis of aminobenzylnaphthols [1]. Now,this process has been known as the Betti reaction and the aminonaphthol product known as a Betti base [2]. The phenolic hydroxyl and amino groups in Betti bases can be used in synthetic building blocks. Aminonaphthols have several interesting biological applications,such as antibacterial,hypotensive,and bradycardiac activities [3, 4, 5]. Optically active Betti bases can be used as ligands to chelate with organometallic reagents in different reactions to provide highly efficient asymmetric reaction [6, 7]. The classical synthesis of Betti bases generally involves a modified Mannich pathway by the condensation of 2-naphthol,aldehydes,and amines. However, various modifications have been made to prepare Betti base derivatives by using other naphthols,quilinols,and alkylamines [8, 9, 10, 11]. In recent years,several more convenient and green procedures for Betti reactions have also been successfully developed [12, 13, 14, 15, 16, 17, 18, 19]. However,there is nearly no reports about using ketone to replace aldehyde in the Betti reaction. In continuation of our ongoing effort to develop new environmentally benign multicomponent reactions [20, 21, 22],herein we report the three-component reaction of β-naphthol,cyclic amines and isatins for the convenient synthesis of new type of Betti bases. 2. Experimental
General procedure for the preparation of compounds 1a-1p :A mixture ofβ-naphthol (2.0 mmol),cyclic amines (2.5 mmol) and isatins (2.0 mmol) in 20 mL methylene dichloride was refluxed for about 24 h. Then the solvent was removed at reduced pressure by rotator evaporation. The residue was titrated with alcohol to give the solid product,recrystallized from ethanol to give the pure product 1a-1p . Some selected data are listed below:
3-(2-Hydroxynaphthalen-1-yl)-3-(piperidin-1-yl)indolin-2-one (1a): White solid,42%,mp 153-156℃; IR (KBr,cm-1 ):ν 3682, 3315,3211,2929,2857,1811,1692,1614,1518,1473,1412,1351, 1277,1231,1174,1104,1033,964,929,859,812; 1H NMR (600 MHz,CDCl3):δ13.97 (s,1H,OH),9.14 (s,1H,NH),7.71-7.67 (m,2H,ArH),7.47-7.44 (m,2H,ArH),7.21-7.17 (m,2H,ArH),7.12- 7.17 (m,2H,ArH),7.12-7.07 (m,2H,ArH),6.96 (t,1H,J= 7.2 Hz, ArH),6.83 (d,1H,J= 7.8 Hz,ArH),3.34 (d,1H,J= 12.0 Hz,CH), 2.91-2.87 (m,1H,CH),2.72 (d,1H,J= 10.2 Hz,CH),2.33 (t,1H, J= 10.2 Hz,CH),1.82-1.75 (m,3H,CH),1.66 (d,1H,J= 13.2 Hz,CH), 1.61-1.54 (m,1H,CH),1.24 (t,1H,J= 7.2 Hz,CH); 13C NMR (150 MHz,CDCl3):δ174.9,158.8,140.1,132.0,130.6,129.8,129.6, 129.3,129.2,126.2,125.6,123.8,122.3,121.8,120.4,112.3,110.4, 73.9,48.3,46.2,26.8,26.4,24.2; HRMS (ESI) Calcd. for C23H22KN2O2([M+K]+ ): 397.1313. Found: 397.1310
1-Benzyl-3-(2-hydroxynaphthalen-1-yl)-3-(piperidin-1-yl)indolin-2-one (1b): Light yellow solid,85%,mp 144-147℃; IR (KBr, cm-1 ):ν 3681,3048,2936,2858,1812,1713,1606,1515,1474, 1447,1345,1273,1237,1206,1162,1104,1074,1032,966,925, 859,813; 1H NMR (600 MHz,CDCl3):δ13.97 (s,1H,OH),7.65-7.61 (m,2H,ArH),7.55 (d,2H,J= 7.2 Hz,ArH),7.47 (d,1H,J= 7.2 Hz, ArH),7.41 (t,2H,J= 7.2 Hz,ArH),7.36 (t,1H,J= 7.2 Hz,ArH),7.22 (t, 1H,J= 7.2 Hz,ArH),7.13 (d,1H,J= 9.0 Hz,ArH),7.08 (t,1H, J= 7.2 Hz,ArH),7.00 (d,1H,J= 8.4 Hz,ArH),6.94 (d,2H,J= 7.2 Hz, ArH),6.85 (t,1H,J= 7.8 Hz,ArH),5.17 (d,1H,J= 15.0 Hz,CH),5.00 (d,1H,J= 15.0 Hz,CH),3.32 (d,1H,J= 12.0 Hz,CH),2.90 (t,1H, J= 12.0 Hz,CH),2.65 (d,1H,J= 10.2 Hz,CH),2.16 (t,1H,J= 10.8 Hz, CH),1.79-1.73 (m,3H,CH),1.61 (d,1H,J= 12.6 Hz,CH),1.57-1.51 (m,1H,CH),1.23 (t,1H,J= 7.2 Hz,CH); 13C NMR (150 MHz,CDCl3): δ 172.3,158.7,142.5,135.5,131.8,130.6,129.7,129.2,129.1, 129.1,129.0,128.7,128.2,126.0,125.5,123.8,122.4,122.3,120.4, 112.9,109.2,73.5,48.2,46.4,44.3,26.8,26.4,24.3; HRMS (ESI) Calcd. for C30H28N2NaO2([M+Na]+ ): 471.2043. Found: 471.2039.
1-Butyl-3-(2-hydroxynaphthalen-1-yl)-3-(piperidin-1-yl)indolin-2-one (1c): Light yellow solid,74%,mp 149-152℃; IR (KBr, cm-1 ):ν 3684,3060. 2931,2860,1715,1608,1577,1515,1470, 1414,1352,1279,1236,1205,1143,1103,1065,1032,960,925, 856,815; 1H NMR (600 MHz,CDCl3):δ14.06 (s,1H,OH),7.65 (t, 2H,J= 7.2 Hz,ArH),7.47 (d,1H,J= 7.8 Hz,ArH),7.28-7.25 (m,1H, ArH),7.15-7.10 (m,4H,ArH),6.96 (t,1H,J= 7.8 Hz,ArH),6.91 (d, 1H,J= 7.8 Hz,ArH),3.90-3.88 (m,2H,CH2),3.30 (d,1H,J= 12.6 Hz, CH),2.89-2.86 (m,1H,CH),2.64 (d,1H,J= 9.6 Hz,CH),2.14 (t,1H, J= 10.2 Hz,CH),1.89-1.80 (m,2H,CH2),1.78-1.72 (m,3H,CH), 1.62 (d,1H,J= 13.8 Hz,CH),1.58-1.52 (m,3H,CH),1.25-1.21 (m, 1H,CH),1.08-1.05 (t,3H,J= 7.2 Hz,H3); 13C NMR (150 MHz, CDCl3): d172.1,158.8,142.7,132.0,130.5,129.7,129.2,125.8, 125.5,123.5,122.2,120.4,112.9,108.5,73.4,48.2,46.2,40.1,29.5, 26.8,26.4,24.2,20.7,14.0; HRMS (ESI) Calcd. for C27H30KN2O2 ([M+K]+ ): 453.1939. Found: 453.1937.
1-Benzyl-3-(2-hydroxynaphthalen-1-yl)-3-morpholinoindolin-2-one (1i): White solid,77%,mp 159-162℃; IR (KBr,cm-1 ):ν 3681,3058,2964,2927,2859,2763,1949,1797,1707,1608,1516, 1454,1410,1350,1270,1235,1176,1108,1077,1029,967,935, 869,817; 1H NMR (600 MHz,CDCl3):δ13.42 (s,1H,OH),7.69-7.64 (m,2H,ArH),7.57 (s,2H,ArH),7.45-7.39 (m,4H,ArH),7.25 (s,1H, ArH),7.13 (s,2H,ArH),6.98 (s,3H,ArH),6.88 (s,1H,ArH),5.16 (d, 1H,J= 14.4 Hz,CH),5.03 (d,1H,J= 14.4 Hz,CH),3.92 (d,1H, J= 9.6 Hz,CH),3.79 (s,2H,CH2),3.59 (t,1H,J= 9.6 Hz,CH),3.31 (s, 1H,CH),3.08 (d,1H,J= 10.8 Hz,CH),2.45 (t,2H,J= 9.0 Hz,CH2); 13C NMR (150 MHz,CDCl3): d171.9,158.0,142.5,135.4,131.8, 130.9,130.1,129.3,129.1,128.7,128.3,128.1,126.2,125.6,123.9, 122.6,122.4,120.3,111.9,109.4,73.1,67.5,67.3,47.4,46.0,44.3; HRMS (ESI) Calcd. for C29H26KN2O3 ([M+K]+ ): 489.1575. Found: 489.1571.
1-Benzyl-5-chloro-3-(2-hydroxynaphthalen-1-yl)-3-morpholinoindolin-2-one (1o): Yellow solid,82%,mp 168-172℃; IR (KBr, cm-1 ):ν 3682,3062,2967,2892,2849,1893,1815,1719,1604, 1517,1475,1416,1362,1328,1267,1228,1178,1113,1076,1031, 977,940,879,817; 1H NMR (600 MHz,CDCl3):δ13.23 (s,1H,OH), 7.71-7.66 (m,2H,ArH),7.55 (d,2H,J= 7.2 Hz,ArH),7.45-7.39 (m, 4H,ArH),7.24 (t,1H,J= 7.8 Hz,ArH),7.14 (d,2H,J= 7.8 Hz,ArH), 6.96-6.92 (m,3H,ArH),5.15 (d,1H,J= 15.0 Hz,CH),5.01 (d,1H, J= 15.0 Hz,CH),3.92 (d,1H,J= 10.8 Hz,CH),3.82-3.77 (m,2H, CH2),3.64-3.60 (m,1H,CH),3.31 (t,1H,J= 11.4 Hz,CH),3.07 (d, 1H,J= 12.6 Hz,CH),2.49-2.41 (m,2H,CH2); 13C NMR (150 MHz, CDCl3): d171.4,158.0,141.1,134.9,131.6,131.2,130.1,129.8, 129.3,129.2,128.7,128.4,126.3,125.9,122.7,122.1,120.3,111.3, 110.4,73.0,67.4,67.3,47.4,46.1,44.5; HRMS (ESI) Calcd. for C29H25ClKN2O3([M+K]+ ): 523.1185. Found: 523.1183.
1-Butyl-5-chloro-3-(2-hydroxynaphthalen-1-yl)-3-morpholinoindolin-2-one (1p): White solid,71%,mp 161-163℃; IR (KBr, cm-1 ):ν 3682,3061,2961,2863,1905,1706,1608,1516,1481, 1338,1268,1235,1191,1149,1114,989,940,886,824; 1HNMR (600 MHz,CDCl3):δ13.27 (s,1H,OH),7.72-7.69 (m,2H,ArH),7.46 (s,1H,ArH),7.28 (d,1H,J= 8.4 Hz,ArH),7.21-7.14 (m,3H,ArH), 7.09 (d,1H,J=8.4Hz,ArH),6.88(d,1H,J= 8.4 Hz,ArH),3.93-3.87 (m,3H,CH),3.84-3.77 (m,2H,CH2),3.63 (t,1H,J= 12.0 Hz,CH), 3.28 (t,1H,J= 11.4 Hz,CH),3.05 (d,1H,J= 12.6 Hz,CH),2.48-2.42 (m,2H,CH2),1.88-1.80 (m,2H,CH2),1.58-1.52 (m,2H,CH2),1.07 (t,3H,J= 7.8 Hz,H3); 13CNMR(150MHz,CDCl3):δ171.3,158.0, 141.3,131.7,131.2,130.2,129.4,129.3,129.0,126.2,125.9,122.7, 122.0,120.4,111.3,109.7,72.9,67.4,67.3,47.3,45.9,40.3,29.5, 20.6,13.9; HRMS (ESI) Calcd. for C26H27ClKN2O3 ([M+K]+ ): 489.1342. Found: 489.1339. 1H NMR and 13C NMR spectra of all compounds and others data are available in Supporting information. 3. Results and discussion
Initially,the reaction conditions were examined using bnaphthol,piperidine andN-benzylisatin as a model reaction. We found that the reaction at room temperature proceeded much more slowly in ethanol,acetonitrile,tetrahydrofuran or toluene than in methylene dichloride. Next,we examined this reaction using different bases as catalyst. Triethylamine,DABCO and piperidine all led to the good yields of the product. Thus,it is convenient to use a slight excess piperidine both as reactant and base catalyst for this reaction. At last,we found that the reaction was finished in reflux CH2Cl2 for 24 h to give the expected 1-benzyl-3-(2-hydroxynaphthalen-1-yl)-3-(piperidin-1-yl)indolin-2-one (1b) in 85% yield.
Under the optimized reaction conditions,except isatin afforded product 2a in moderate yield (Table 1,entry 1),other isatins with N-benzyl orN-n-butyl groups all reacted smoothly to give products 1b-1h in good yields (Table 1,entries 2-8). In addition,another common cyclic amine,morpholine,was also utilized in the reaction. The expected 3-(2-hydroxynaphthalen-1-yl)-3-morpholinoindolin-2-ones 1i-1p were also obtained in satisfactory yields (Table 1,entries 9-16). The reaction with isatin itself still afforded product 1k in lower yield.
| Table 1 Synthesis of Betti bases 1a-1p from three-component reaction.a |
In order to develop the scope of this reaction,other secondary amines,such as pyrrolidine,dimethylamine,diethylamine,di(npropyl)amine,were also tested in the reaction. It is very disappointed to find that no expected Betti bases were formed in the reactions. On the other hand,the similar reactions containinga-naphthol,resorcinol,and pyrogallol did not afforded the expected products. These facts indicated that this reaction is very sensitive to the structures of the substrates. The formation mechanism of the compounds 1a-1p is almost certainly the traditional Mannich-type reaction process (Scheme 1),which contains the first formation of active iminium ion (A) from reaction of isatin with piperidine,subsequent nucleophilic attack of the carbon atom ofβ-naphthol on the iminium ion and a hydride shift process (B).

|
Download:
|
| Scheme 1.Proposed reaction mechanism for three-component reaction. | |
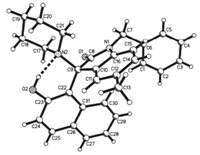
The structures of the prepared Betti bases 1a-1p were fully characterized with IR,HRMS, 1H NMR and 13C NMR spectroscopy. In the 1H NMR spectra of the 1a-1p ,the methylene units in Nbenzyl group and in the piperidyl or morpholinyl ring all display the magnetically anisotropic absorption. As for an example,the 1H NMR spectrum of 1d displays a singlet at 13.92 ppm for the phenolic hydroxy group. The methylene unit in benzyl group showed two doublets at 5.13,5.04 ppm with a germinal coupling constant J= 14. 4 Hz. The two NCH2 units in piperidyl group appears in four doublets with germinal coupling constantsJ= 11. 4 or 12.0 Hz. The single crystal structure of the compound 1d was determined by X-ray diffraction method (Fig. 1),and the crystallographic data has been deposited at the Cambridge Crystallographic Database Centre (CCDC 969319). There is one intramolecular H-bond between the phenolic hydroxy group and nitrogen atom of pipridyl ring. Obviously,due to existence of the intramolecular H-bond,the methylene units in the molecule showed stronger magnetically anisotropic property.
|
Download:
|
| Fig. 1. Molecular structure of compound 1d. | |
In summary,we investigated three-component reaction of bnaphthol,cyclic amines and isatins and found the convenient synthetic protocol for the new type of Betti bases. The oxindole moiety was successfully incorporated into the Betti bases for the first time. This result not only provided a new multicomponent reaction for the synthesis of versatile indolinone compounds,but also opened a new way for the further development of the wellknown Betti reaction. The potential uses of the reaction in synthetic and medicinal chemistry might be quite significant.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (No. 21272200) and the Priority Academic Program Development of Jiangsu Higher Education Institutions. We also thank the Analysis and Test Center of Yangzhou University providing instruments for analysis.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.11.009.
| [1] | M. Betti, β-naphthol phenylaminomethane, Org. Synth. Coll. 1 (1941) 381-384. |
| [2] | C. Cardellicchio, M.A. Capozzi, A. Faso, The Betti base: the awakening of a sleeping beauty, Tetrahedron: Asymmetry 21 (2010) 507-517. |
| [3] | B.P. Mathew, A. Kumar, S. Sharma, P.K. Shukla, M. Nath, An eco-friendly synthesis and antimicrobial activities of dihydro-2H-benzo-and naphtho-1,3-oxazine derivatives, Eur. J. Med. Chem. 45 (2010) 1502-1507. |
| [4] | M. Salamone, R. Amorati, S. Menichetti, C. Viglianisi, M. Bietti, Structural and medium effects on the reactions of the cumyloxyl radical with intramolecular hydrogen bonded phenols. The interplay between hydrogen-bonding and acid-base interactions on the hydrogen atom transfer reactivity and selectivity, J. Org. Chem. 79 (2014) 6196-6205. |
| [5] | P.F. Kaiser, J.M. White, C.A. Hutton, Enantioselective preparation of a stable boronate complex stereogenic only at boron, J. Am. Chem. Soc. 130 (2008) 16450-16451; G. Cheng, X. Wang, R. Zhu, et al., Total synthesis of (-)-cocaine and (-)-ferruginine, J. Org. Chem. 76 (2011) 2694-2700. |
| [6] | X. Wang, Y. Dong, J. Sun, et al., Nonracemic Betti base as a new chiral auxiliary: application to total syntheses of enantiopure (2S,6R)-dihydropinidine and (2S,6R)-isosolenopsins, J. Org. Chem. 70 (2005) 1897-1900. |
| [7] | H. Wei, L. Yin, H. Luo, X. Li, A.S.C. Chan, Structural influence of chiral tertiary aminonaphthol ligands on the asymmetric phenyl transfer to aromatic aldehydes, Chirality 23 (2011) 222-227. |
| [8] | C. Cimarelli, D. Fratoni, A. Mazzanti, G. Palmieri, Enantiopure a-imino glyoxylate: a versatile substrate for the spontaneous asymmetric synthesis of unnatural hydroxyaryl glycinates, Tetrahedron: Asymmetry 22 (2011) 591-596. |
| [9] | J. Lu, X. Xu, C. Wang, et al., Synthesis of chiral ligands derived from the Betti base and their use in the enantioselective addition of diethylzinc to aromatic aldehydes, Tetrahedron Lett. 43 (2002) 8367-8369. |
| [10] | I. Szatmári, F. Fülöp, Microwave-assisted one-pot synthesis of (aminoalkyl)-naphthols and (aminoalkyl)quinolinols by using ammonium carbamate or ammonium hydrogen carbonate as solid ammonia source, Synthesis (2009) 775-778. |
| [11] | I. Szatmári, F. Fülöp, Syntheses and transformations of 1-(a-aminobenzyl)-2-naphthol derivatives, Curr. Org. Synth. 1 (2004) 155-165. |
| [12] | R. Csutortoki, I. Szatmári, F. Fülöp, Syntheses of amido-, carbamido-and carbamatoalkylnaphthols, Curr. Org. Synth. 10 (2013) 564-583. |
| [13] | I. Szatmári, F. Fülöp, Syntheses, transformations and applications of aminonaphthol derivatives prepared via modified Mannich reactions, Tetrahedron 69 (2013) 1255. |
| [14] | S.D. Dindulkar, V.G. Puranik, Y.T. Jeong, Supported copper triflate as an efficient catalytic system for the synthesis of highly functionalized 2-naphthol Mannich bases under solvent free condition, Tetrahedron Lett. 53 (2012) 4376-4380. |
| [15] | A. Kumar, A. Saxena, M. Dewan, A. De, S. Mozumdar, Recyclable nanoparticulate copper mediated synthesis of naphthoxazinones in PEG-400: a green approach, Tetrahedron Lett. 52 (2011) 4835-4839. |
| [16] | B. Karmakar, J. Banerji, A competent pot and atom-efficient synthesis of Betti bases over nanocrystalline MgO involving a modified Mannich type reaction, Tetrahedron Lett. 52 (2011) 4957-4960. |
| [17] | A. Kumar, M.K. Gupta, M. Kumar, Non-ionic surfactant catalyzed synthesis of Betti base in water, Tetrahedron Lett. 51 (2010) 1582-1584. |
| [18] | M. Shafiee, A.R. Khosropour, I. Mohammadpoor-Baltork, et al., An efficient, expeditious, and diastereoselective one-pot pseudo-five-component reaction for the synthesis of new bis-Betti bases under catalyst-free conditions, Tetrahedron Lett. 53 (2012) 3086-3090. |
| [19] | M. Kidwai, R. Chauhan, Catalyst-free synthesis of Betti bases in a Mannich-type reaction, Asian J. Org. Chem. 2 (2013) 395-398. |
| [20] | J. Sun, Y. Sun, H. Gong, Y.J. Xie, C.G. Yan, Facile synthesis of dispirooxindole-fused heterocycles via domino 1,4-dipolar addition and Diels-Alder reaction of in situ generated Huisgen 1,4-dipoles, Org. Lett. 14 (2012) 5172-5175. |
| [21] | J. Sun, Y. Sun, H. Gao, C.G. Yan, Synthesis of spiro[indoline-3,20-quinoline] derivatives through a four-component reaction, Eur. J. Org. Chem. (2012) 1976-1983. |
| [22] | H. Gong, J. Sun, C.G. Yan, Synthesis of triphenylphosphanylidene spiro[cyclopent[2]ene-1,30-indolines] with three-component reaction of triphenylphosphine, dialkyl acetylenedicarboxylates and 3-phenacylideneoxindoles, Synthesis (2014) 489-495. |