Fluoride anion has distinctive features than other anions due to its exceptional electronegativity and small radius [1]. Based on these unique characteristics,the fluoride anion plays an important role in biological,clinical and environmental fields,especially the function of preventing dental caries and the treatment of osteoporosis [2, 3, 4, 5]. Meanwhile,fluoride overload is harmful to human health,which can cause fluorosis [6]. Excessive fluoride anion is also considered as potential danger to the environment [7]. Accordingly,the development of efficient probes for fluoride anion has attracted much more attention. A large number of synthetic fluorophores have been designed as chemosensors for F- in the past decade. However,some sensing systems suffer from several typical drawbacks,such as low limit of detection,poor selectivity for anions,difficulty of synthesis,and impossible recognition by the naked-eye [8, 9, 10].
It is well-known that the imidazole unit,due to the acidity of the N-H proton,acts as an excellent hydrogen bond donor in the molecular structure,which can be utilized in detecting fluoride. Tamiaki et al. [11] and Liu et al. [12] reported a series of imidazo[4, 5, f]-1,10-phenanthroline metal-complexes as colorimetric and fluorescence chemosensors for fluoride anions, respectively. The results proved that the imidazolyl N-H could be hydrogen-bonded upon the addition of F- and results in the suppression of the photosensitization. These chemosensors were mainly designed on the coordination of the metal center (Eu,Re,Pt) with imidazolyl chromophores,which have the disadvantage of complicated preparation and relative high cost. In addition, phenazine derivatives have been widely used in the field of polymer conductive materials,exhibiting excellent luminescent properties and photophysical stabilities [13, 14, 15, 16]. Zhang’s group [17] reported a heteroacene based F- sensor,2-(2,3,4,5-tetrafluorophenyl)-1H-imidazo[4, 5, b]phenazine,which shows selectivity and multiple responses to F- through combined hydrogen bonding,deprotonation,and anion-π interactions. However,the studies on the phenazine based F- sensors are still rare and the low detection limits prohibit their extensive application.
In this work,we designed and synthesized a new probe based on imidazole and phenazine structures (Scheme 1). The Br substituent was introduced with the aim of improving the selectivity and sensitivity for F- due to its moderate electronegativity. At the same time,the synthesis of this probe is simpler than other probes. The colorimetric and fluorescent characteristics of this chemosensor drastically change with the addition of fluoride anions in DMSO solution. Furthermore,paper test strips of this probe were prepared to detect F- in water-solvent system.

|
Download:
|
| Scheme 1.Synthetic route for probe 1. | |
All reagents were obtained from commercial suppliers and used without further purification,unless otherwise indicated. The 1H (400 MHz) NMR and 13C NMR (100 MHz) spectra were recorded in DMSO-d6at room temperature using a Bruker Ultra Shield Plus 400 MHz instrument with tetramethylsilane (TMS) as internal reference. Melting points (m.p.) were measured on a Gal-lenkamp apparatus. Mass spectrometry analyses were acquired using MAT-212 instrument. Fluorescence spectra were collected using a Perkin-Elmer LS 50B spectrofluorometer. UV-vis absorption spectra were recorded using an Agilent 8453 spectrophotometer.
Synthesis of probe 1: Both 2,3-diamino-phenazine (0.21 g, 1 mmol) and 4-bromobenzaldehyde (0.19 g,1 mmol) were dissolved to DMF (8 mL). The solution was stirred under reflux for 16 h. After cooling to room temperature,the brown precipitate was filtered,washed with hot ethanol three times,then recrystallized with DMF-H2O to get a brown powdery product 1(0.29 g,78% yield). M.p.>300℃, 1H NMR (DMSO-d6,400 MHz):δ7.89 (q,4 H, J= 8.0 Hz),8.23 (q,2H.J= 8.0 Hz),8.32 (d,2H,J= 8.0 Hz),8.39 (s, 2H),13.51 (s,1H), 13C NMR (DMSO-d6,100 MHz):d158.87,147.67, 141.34,139.87,131.90,129.72,128.93,128.80,124.62,116.87, 109.88. ESI-MSm/z:(M-1)+ calcd. for C19H11N4Br 374.02; found 372.9.
All UV-vis spectroscopy were recorded after the addition of tetrabutylammonium salts in DMSO [18, 19],while keeping the ligand concentration constant (1×10-5 mol/L) on a Agilent 8453 spectrophotometer. The solutions of the anions were prepared from the tetrabutylammonium salts of F- ,Cl- ,Br- ,I- OAc- , H2PO4- ,HSO4- and ClO4- .
All fluorescence spectroscopy were recorded after the addition of tetrabutylammonium salts in DMSO,while keeping the ligand concentration constant (1×10-5 mol/L),on a Perkin-Elmer LS50B spectrofluorometer. The solutions of the anions were prepared from the tetrabutylammonium salts of F- ,Cl- ,Br- ,I- OAc- , H2PO4- ,HSO4- and ClO4- .
For 1H NMR titrations,probe 1 was dissolved in DMSO-d6, which was mixed different equiv. of F- in NMR tubes. The spectra were performed at 298 K [20]. 3. Results and discussion
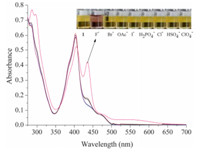
The selective properties of probe 1(1×10-5 mol/L) in DMSO solution toward various anions were first evaluated. Fig. 1 illustrates the absorption spectra changes and color changes when probe 1in DMSO solution was added to 5 equiv. of the F- and 20 equiv. of other anions. Absorption spectra show remarkable changes that the original absorption peak at 405 nm red-shift to 435 nm after adding F- ,while a broad absorption band at 550 nm appears. Furthermore,no obvious changes were observed after the addition of other halogen anions,such as Cl- and Br- ,I- and more complicated anions,such as OAc- ,H2PO4- ,HSO4- and ClO4- . Simultaneously,when F- (5 equiv.) was added to the DMSO solution of probe 1(1×10-4 mol/L),the color of the solution changed from yellow to red. This could be clearly observed by the naked-eye. The fluorescence of probe 1in DMSO solution also exhibits significant differences upon adding various anions. When F- was added to the solution of probe 1(1×10-5 mol/L),the intensity of the emission band at 530 nm decreased and a new emission band appeared at ca. 650 nm. The fluorescent color changed from green to red under a UV lamp (365 nm light irradiation),which could be distinguished by the naked-eye. Meanwhile,none of the other anions induced any significant changes in the fluorescence intensity at 530 nm compared with F- (Fig. S5 in Supporting information).
|
Download:
|
| Fig. 1. UV-vis absorption spectra of probe 1 (1×10-5mol/L) in DMSO,uponaddition of F-(5.0 equiv.) and other anions (20.0 equiv of Cl-,Br-,I-,OAc-,H2PO4-,HSO4-and ClO4-as their TBA salts). Inset illustration: color change of sensor(1×10-4mol/L) in DMSO after addition of 5 equiv. of different anions. From left toright: a solution of probe1and those after the addition of anions (as TBA salts): F-,Br-,OAc-,I-,H2PO4-,Cl-,HSO4-and ClO4-. | |
A spectrophotometric titration method was used to investigate the interaction between probe 1 and F- . When tetrabutyl ammonium fluoride (TBAF) in THF (3×10-2 mol/L) was added to the solution of probe 1(1×10-5 mol/L),the absorption band at 405 nm gradually red-shifted and the absorption intensity decreased (Fig. 2a). Meanwhile,a new absorption band at 435 nm emerged and its absorption intensity significantly increased with the addition of F- . When 11 equiv. of F- were added,the absorption bands at 405 nm and 435 nm approached saturation,which is due to the deprotonation of the N-H in the imidazolyl moiety [21]. A well-defined isosbestic point appeared at 411 nm. Minor changes of the absorption spectra occurred when the ratio of F- to probe 1was less than three,which implies the existence of a ground state F- ···H-N interaction [21]. However, when the range of this ratio was adjusted from 4 to 8,significant changes occurred in absorbance at 405 nm and 435 nm and exhibited a near linear relationship. In addition,the Job’s plot indicates that a 1:1 hydrogen-bonded complex formed between probe 1and F- (Fig. S6 in Supporting information) [22]. The binding constant Ka was determined as 3.3×103 L/mol (Fig. S7 in Supporting information) [23].

|
Download:
|
| Fig. 2.(a) UV-vis absorption titration of probe 1(1×10-5 mol/L) in DMSO solution (range of 0-12 equiv. of fluoride ions). Inset illustration: correlation curve of probe 1at 435 nm (black) and 405 nm (red) adding different equivalents of F- . (b) Fluorescence titration of probe 1in DMSO solution (1×10-5 mol/L) (range of 0-12 equiv. of fluoride ions). Inset illustration: correlation curve of probe 1at 530 nm (black) and 650 nm (red) adding different equivalents of F-. | |
Fluorescence titrations with F- in varying concentrations were conducted. As shown in Fig. 2b,with the concentration of F- increased,the emission band of probe 1in DMSO solution at 530 nm gradually diminished. The fluorescence of probe 1was essentially quenched by 11 equiv. of F- . Meanwhile,a new emission band appeared at 650 nm,which shows the same trend found for the UV-vis changes. The near-linear correlation curves of probe 1at 530 and 650 nm (vs.concentration of F- in DMSO) demonstrated the potential utility of probe 1for quantitative determination of F- in DMSO. Furthermore,the detection limit of the probe 1for the determination of F- was estimated to be 6.2×10-6 mol/L [24]. It is worth noting that compared to the 1Himidazo[4, 5, b]phenazine based F- sensor with 2,3,4,5-tetrafluorophenyl substituent (8.6×10-5 mol/L) [17],the detection limitation of probe 1for F- has been greatly improved.
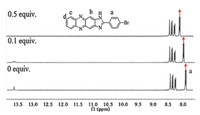
The mechanism of the interaction between probe 1and F- was investigated by 1H NMR at room temperature,as shown in Fig. 3. The peak of N-H of the imidazoyl moiety appears at low-field without addition of F- ,indicating the N-H has a relative high acidity and is easy to form hydrogen bonds [25]. When 0.1 equiv. of F- was added,the peak of N-H became weaker and broader proving the presence of hydrogen bonding interaction between the fluoride and the proton of N-H. After addition of 0.5 equiv. of F- , the proton signals of the N-H entirely disappeared,which is in good agreement with the formation of strong hydrogen-bonded complexes with F- . In addition,F- also caused the protons Ha of probe 1(nearest to the imidazole ring) to shift to lower field and polarizes the C-H bonds in proximity to the imidazole nitrogen, creating a partial positive environment at Ha [26].
|
Download:
|
| Fig. 3.1 HNMR spectra measured by titration of a DMSO-d6solution of probe1with 0 equiv.,0.1 equiv. and 0.5 equiv. of [Bu4N]F. | |
To date,most of the sensors reported in literatures were utilized in the detection for F- in the organic phase,thus the design of a probe for use in aqueous media is still valuable [27]. Bearing this fact in mind,detecting F- in an organic solvent-water system was initiated. In order to evaluate the practical application of probe 1, test strips were prepared by immersing filter papers into a DMSO solution of probe 1(1×10-4 mol/L) and then dried in air [28]. As shown in Fig. 4,when F- was added on the test strips,the obvious color change could be observed under the 365 nm UV lamp compared with other anions. Meanwhile,non-interference experimental results indicate the paper test strips can still detect F- (c=1×10-3 mol/L) efficiently in the presence of other anions at high concentrations (c=20×10-3 mol/L). Although the emission of probe 1was quenched in the aqueous system,the test strips could conveniently sense F- in water-solvent solutions. Color change of the test strips in THF solutions of F- can be observed when the volume ratio of organic solvent to water is higher than 10:1 (Fig. S9 in Supporting information). The test strips are also applicable in different organic solvents,such as THF,ethanol, DMSO and acetone (Fig. S10 in Supporting information). In addition,the color of the test strips gradually changed from yellow to orange after immerging into the THF solutions of F- at different concentrations (Fig. S11 in Supporting Information). The detection limit of the test paper system is about 1×10-4 mol/L by the naked-eye (Fig. S11).

|
Download:
|
| Fig. 4.Photographs of probe1on test papers. (a) blank: test paper without anyanions,F(a): test paper immersed into THF/water (20:1) solution with F-,F(b): testpaper dabbing THF solution with F-,from fourth to tenth: Cl-,Br-,I-,OAc-,H2PO4-,HSO4-and ClO4-under the 365 nm UV lamp (the concentration of anionsis 10 mmol/L). (b) Color change of sensor on test papers under the 365 nm UV lamp,after immersion into solutions,which are consisted of 1×10-3mol/L of F-and20×10-3mol/L of other anions. | |
A new colorimetric and fluorimetric chemosensor,based on 1Himidazo[4, 5, b]phenazine (probe 1),has been designed and synthesized,which could be utilized for F- detection. This probe exhibits high selectivity and sensitivity with a detection limitation of 6.2×10-6 mol/L. After the addition of F- to a solution of probe 1, the color of the solution changes from yellow to red and can be reasonably observed by the naked-eye. Furthermore,the use of paper strips,as described,also indicates that probe 1can detect F- in a water-solvent solution. These studies suggest this probe has potential applications in physiological and environmental systems.
AcknowledgmentsThe authors greatly acknowledge the financial support in part by Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No. 13KJB150018),Natural Science Foundation of Jiangsu Province (No. BK20130926),Innovation program of Shanghai institute of technical physics of the Chinese Academy of Sciences (No. 2013,Q-DX-36,Q-DX-37),and Postgraduate Innovation Fund of Jiangsu Province (No. 2013,CXZZ13_0459).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.10.028.
| [1] | P. Ashokkumar, H. Weißhoff, W. Kraus, et al., Test-strip-based fluorometric detection of fluoride in aqueous media with a BODIPY-linked hydrogen-bonding receptor, Angew. Chem. Int. Ed. 53 (2014) 2225-2229. |
| [2] | K.L. Kirk, Biochemistry of the Elemental Halogens and Inorganic Halides, Plenum Press, New York, 1991. |
| [3] | Q. Zhao, F.Y. Li, S. Liu, et al., Highly selective phosphorescent chemosensor for fluoride based on an iridium(III) complex containing arylborane units, Inorg. Chem. 47 (2008) 9256-9264. |
| [4] | J.F. Zhao, G. Li, Q.C. Zhang, et al., A new N-substituted heteroacene can detect CN- and F- anions via anion-p interaction, RSC Adv. 3 (2013) 9653-9657. |
| [5] | G. Li, Y.C. Wu, Q.C. Zhang, et al., Synthesis, physical properties, and anion recognition of two novel larger azaacenes: benzannelated hexazaheptacene and benzannelated N,N'-dihydrohexazaheptacene, Chem. Asian J. 8 (2013) 1574-1578. |
| [6] | B.L. Riggs, Bone and Mineral Research, Annual 2, Elsevier, Amsterdam, 1984. |
| [7] | S. Madhu, M. Ravikanth, Boron-dipyrromethene based reversible and reusable selective chemosensor for fluoride detection, Inorg. Chem. 53 (2014) 1646-1652. |
| [8] | E. Ganapathi, S. Madhu, T. Chatterjee, R. Gonnadeb, M. Ravikanth, Synthesis, structure, spectral, electrochemical and sensing properties of 3-amino borondipyrromethene and its derivatives, Dyes Pigments 102 (2014) 218-227. |
| [9] | T. Liu, H.X. Zhang, X. Zhou, et al., Mechanism of Ir(ppy)2(N^N)+ (N^N = 2-phenyl-1H-imidazo [4,5-f][1,10] phenanthroline) sensor for F-, CF3COOH, and CH3COO-: density functional theory and time-dependent density functional theory studies, J. Phys. Chem. A 112 (2008) 8254-8262. |
| [10] | Y.H. Zheng, C.L. Tan, G.P.C. Drummen, et al., A luminescent lanthanide complexbased anion sensor with electron-donating methoxy groups for monitoring multiple anions in environmental and biological processes, Spectrochim. Acta A 96 (2012) 387-394. |
| [11] | Q.M. Wang, H. Tamiaki, Highly efficient and selective turn-off quenching of ligand-sensitized luminescence from europium imidazo[4,5-f]-1,10-phenanthroline complex by fluoride ion, J. Photochem. Photobiol. A 206 (2009) 124-128. |
| [12] | S.S. Li, C. Zhang, S.Y. Huang, et al., Highly selective colorimetric and fluorescent sensors for the fluoride anion based on imidazo[4,5-f]-1,10-phenanthroline metal-complexes, RSC Adv. 2 (2012) 4215-4219. |
| [13] | Y.F. Xie, T. Fujimoto, S. Dalgleish, et al., Synthesis, optical properties and charge transport characteristics of a series of novel thiophene-fused phenazine derivatives, J. Mater. Chem. 1 (2013) 3467-3481. |
| [14] | F.A. Viva, E.M. Andrade, M.I. Florit, F.V. Molina, Electropolymerization of 2-methoxyaniline. Polymerization kinetics and phenazine insertion at low monomer concentration, Phys. Chem. Chem. Phys. 4 (2002) 2293-2300. |
| [15] | P. Muthirulan, N. Rajendran, Poly (o-phenylenediamine) coatings on mild steel: electrosynthesis, characterization and its corrosion protection ability in acid medium, Surf. Coat. Technol. 206 (2012) 2072-2078. |
| [16] | B.B. Shi, Y.M. Zhang, et al., Recognition of dihydrogen phosphate ions using the cadmium complex of 2-pyridine-1H-imidazo[4,5-b]-phenazine: utilization of the mechanism of twisted intramolecular charge transfer, long wavelength emission, New J. Chem. 37 (2013) 3737-3744. |
| [17] | C.Y. Wang, G. Li, Q.C. Zhang, et al., A novel heteroacene, 2-(2,3,4,5-tetrafluorophenyl)-1H-imidazo[4,5-b] phenazine as a multi-response sensor for F detection, Tetrahedron Lett. 54 (2013) 2633-2636. |
| [18] | Y.Y. Huang, M.J. Wang, Z. Yang, et al., High efficient probes with Schiff base functional receptors for hypochlorite sensing under physiological conditions, Chin. Chem. Lett. 25 (2014) 1077-1081. |
| [19] | Q. Liu, L. Xue, D.J. Zhu, et al., Highly selective two-photon fluorescent probe for imaging of nitric oxide in living cells, Chin. Chem. Lett. 25 (2014) 19-23. |
| [20] | M.R. Rao, M. Shaikh, M. Mobin, Boron-dipyrromethene based specific chemodosimeter for fluoride ion, Tetrahedron 66 (2010) 1728-1734. |
| [21] | L.Y. Zhao, G.K. Wang, Y. Zhou, 1,8-Naphthalimide-based visible colorimetric sensor for the selective sensing of fluoride, acetate and hydroxyl anions, J. Fluor. Chem. 158 (2014) 53-59. |
| [22] | G.Y. Gao, W.J. Qu, B.B. Shi, A highly selective fluorescent chemosensor for iron ion based on 1H-imidazo [4,5-b] phenazine derivative, Spectrochim. Acta A 121 (2014) 514-519. |
| [23] | S.J. Liu, Z.J. Shi, W.J. Xu, et al., A class of wavelength-tunable near-infrared aza-BODIPY dyes and their application for sensing mercury ion, Dyes Pigments 103 (2014) 145-153. |
| [24] | W.Y. Lin, L. Yuan, Z.M. Cao, et al., A sensitive and selective fluorescent thiol probe in water based on the conjugate 1,4-addition of thiols to α,β-unsaturated ketones, Chem. Eur. J. 15 (2009) 5096-5103. |
| [25] | S. Madhu, M. Ravikanth, Boron-dipyrromethene based reversible and reusable selective chemosensor for fluoride detection, Inorg. Chem. 53 (2014) 1646-1653. |
| [26] | R.M.F. Batista, P.G. Costa, Naphthyl-imidazo-anthraquinones as novel colorimetric and fluorimetric chemosensors for ion sensing, J. Photochem. Photobiol. A 259 (2013) 33-40. |
| [27] | S. Turan, E.U. Akkaya, Chemiluminescence sensing of fluoride ions using a selfimmolative amplifier, Org. Lett. 16 (2014) 1680-1683. |
| [28] | H. Lu, Q.H. Wang, Z.F. Li, A specific chemodosimeter for fluoride ion based on a pyrene derivative with trimethylsilylethynyl groups, Org. Biomol. Chem. 9 (2011) 4558-4562. |




