b State Key Laboratory of Natural and Biomimetic Drugs, Peking University, Beijing 100191, China
Since organic light emitting materials have wide application,it is important to develop highly efficient emitters. Among them, conjugated organic compounds with good photoluminescence responses are promising candidates for optoelectronic use. They present advantages such as low cost and ease of processing compared to inorganic ones. Moreover,the employment of functional groups endows them with a variety of unique optoelectronic properties [1, 2, 3]. However,emission of these compounds usually becomes quite weak in solid state due to dipole-dipole interaction [4],intermolecular energy and electron transfer [5] and nonradiative decay. This phenomenon is well known as the aggregation caused quenching (ACQ). It greatly inhibits the application of organic luminescent materials. Researchers have been seeking materials with high emission efficiency in solid (aggregated) state. Tanget al.and Parket al.have reported some materials with aggregation induce emission [6].
Besides these,the building of electron donor-acceptor structure is an effective way to improve luminescent property of fluorophores [7, 8, 9]. It can inhibit the fluorescence quenching. Triphenylamine (TPA) is frequently employed to generate various compounds with electron donor-acceptor structure.
Wang’s group reported a triphenylamine-substituted chromophore for non-linear optical applications [10]. Yu developed a 2-(4-bromophenyl)-3-(4-diphenylaminophenyl) acrylonitrile compound emitting green fluorescence in CH2Cl2and in the solid state with good thermal stability [11]. Teulade-Fichou and co-workers synthesized vinyl-pyridinium TPA molecule,a novel far red emitter with high photostability and two photon absorption properties [12]. Fu studied the no-linear and two photon absorption properties of multi-branched and dendritic TPA architectures and found that these chromophores are potential materials for use as broadband and rapid-responsive optical limiters [13]. Tian’s group reported a new class of TPA derivatives with aggregation induced emission colors [14]. Perry and Kinstle both build the donor-acceptor TPA systems to investigate their optical properties [15, 16].
Here in this paper,we have developed a type of donor-acceptor structure based on TPA: triphenylamine pyridine acetonitrile compoundsPyA1-3are synthesized by the coupling of formyltriphenylamines with pyridine acetonitrile. They have large Strokes shift and display bright luminescence in the solid state. Interestingly,compared with other pH probes which respond to decreasing pH values [17, 18, 19, 20, 21],thesePyAcompounds can sense increasing pH values (from pH 2 to pH 7) through a fluorescence turn-on manner. The switched color and fluorescent changes can be observed in solution and 1% agarose gel by naked eyes. The mechanism is proposed to be the TICT effect (Twisted intramolecular charge transfer). Through molecular calculation,it is found that after binding with protons the molecular plane of electronacceptor (pyridinium unit) is perpendicular to the electron donor (TPA) in the first excited state,which destroys the D-π-A structure of PyAs and quenches the fluorescence. Upon deprotonation, fluorescence is switched on.PyA1-3can rapidly respond to pH changes in solution and cell environment. 2. Experimental 2.1. Materials and equipment
All solvents and reagents were commercially available and used without further purification unless for special needs. Parent stock solutions ofPyA1,PyA2andPyA3(10 μmol/L) were prepared in DMSO. PBS buffers with pH 2-7 were prepared and confirmed by pH meter.
1H NMR and 13C NMR spectra were recorded on Varian Mercury 300 spectrometers,using CDCl3as solvent and tetramethylsilane (TMS) as internal reference,respectively. HRMS were recorded on a Brucker APEX IV (7.0 T) and IonSpec 4.7 Tesla FTMS. 2.2. Synthesis
Compounds1-3were synthesized by the literature method [22] and the spectra data are detailed in Supporting information. Synthesis of PyA1-3: We synthesized the PyA1and PyA2 according to our previous reported,in which the methylatedPyA1 andPyA2can detect anti-parallel G-quadruplex structure [23].
Synthesis of PyA3: 4,4,4-Triformyltriphenylamine (329 mg, 1 mmol) and 4-pyridylacetonitrile (389 mg,3.3 mmol) was dissolved in dry CH3OH (2 mL),then potassiumtert-butylate (369 mg, 3.3 mmol) was added (Scheme 1). Then the mixture was stirred for 4 h at r.t. and filtered under reduced pressure to give a red solid of PyA3(377 mg,60%).
|
Download:
|
| Scheme 1.General procedure for the synthesis of PyA1-3. | |
1H NMR (300 MHz,CDCl3):δ8.65 (d,6H, J= 4.8 Hz),7.94 (d,6H,J= 8.1 Hz),7.67 (s,3H),7.55 (d,6H, J= 4.2 Hz),7.25 (d,6H,J= 7.2 Hz). 13C NMR (75 MHz,CDCl3):δ 150.4,148.3,143.5,141.6,131.4,128.6,124.4,119.6,117.1, 107.4. HRMS (ESI) calcd. for C42H28N7[M+H]+ : 630.2406; found: 630.2400. 2.3. Spectroscopic measurements
Absorption spectra were recorded in 1 cm×1 cm quartz cuvettes on Shimadzu UV-2550 spectrophotometer. Fluorescent emission spectra were collected from 480nm to 700 nm on PerkinElmer LS 55. The excitation and emission slit widths were set at 15/10 nm for PyA1,10/5 nm for PyA2 and 10/10 nm for PyA3. Quartz cuvette with 2 mL volume was used for emission measurements. Unless otherwise specified,all spectra were taken at an ambient temperature in 10 μmol/L potassium phosphate buffered 2.4. Acid-base cycle experiment
For pH-switched experiment ofPyA1-3,experimental details were shown in Table S1. To avoid dilution effect,paralleled samples were prepared. The ‘‘Cycle 0’’ means that the dye molecules were first put in deionized water without any acid and base. This aqueous mixture was prepared by adding an aliquot of concentrated DMSO stock solution ofPyAsinto a large volume of water. From No. 1 to No. 10,base and acid were added alternatively to adjust the pH value. Additional water,if needed,was added to ensure that the final dye concentrations of all samples were identical. 2.5. Theoretical calculations
Molecular modeling experiments were carried out to investigate the recognition ofPyAswith protons. The geometries of the host and host-guest complex were optimized using molecular mechanics method with TD-CAM-B3LYP functional. The molecular dynamics simulation package Gaussian 09 program was used for calculations [24]. 2.6. Agarose gel visualization
For image on agarose gel,10μmol/L solution of eachPyA compound was used. 1 wt% agarose gel was prepared using these solutions. The gel was immersed into pH 2 and 7 buffer solutions for visualization. 2.7. Cell culture and confocal imaging
Hela cells were cultured in MEM (Hyclone,China) supplemented with 10% FBS (Hyclone) and penicillin (100 units/mL). The cells were incubated at 37℃ in a 95% humidified atmosphere containing 5% CO2. For the experiment,Hela cells were cultured overnight in 35-mm glass-bottomed dishes (Nest). Then the cells would be first incubated withPyA1-3(10μmol/L) in pH 7 PBS buffer (Hyclone) containing 1/1000 DMSO at 37℃ for 4 h,washed with pH 7 PBS buffer three times,and mounted on the microscope stage for imaging. Then,pH 2 PBS buffer was used to replace pH 7 buffer and incubated with cells for 5 min to get the images. Confocal images were taken using Carl Zeiss LSM 710. The excitation wavelengthes ofPyA1-3were 440,460,465 nm and emissions were 550,560,570 nm (green fluorescence). 3. Results and discussion 3.1. Optical properties
Different formyl-triphenylamines were synthesized in one step reaction and they were coupled with 4-pyridinium acetonitrile to generate thePyA1,PyA2andPyA3,as shown in Scheme 1. Their optical properties were studied. As can be seen from Fig. S1 (Supporting information),PyA1,PyA2andPyA3displayed bright light emitting in the solid state under UV irradiation,indicating that they were good light emitting materials because they overcame the fluorescence quenching effect of compounds in solid state.PyA1-3 were soluble in common organic solvent and can be dispersed in water showing strong fluorescence. The excitation and emission wavelength were investigated (Table 1). In less polar solvent like cyclohexane,the maximum emission wavelengths ofPyA1-3were the shortest. The fluorescence wavelengths were red-shifted with increasing solvent polarity and displayed the longest emission in more polar solvents such as DMSO,acetonitrile and DMF. Due to the interaction of solvent molecule with compound dipole,energy of the ground state and excited state were influenced,leading to the wavelength shift in fluorescence emission spectra.
| Table 1 Excitation and emission wavelengths ofPyA1-3 in water and organic solvents. |
We recorded the fluorescence spectra ofPyA1andPyA2in solvent mixtures. As shown in Fig. 1,PyA1showed relatively lower FL intensity in pure acetonitrile and ethanol. When water fraction increased to 60%,FL intensity decreased rapidly. The maximum emission wavelengths were shifted and FL intensity was enhanced when water fraction was above 60%. The FL intensities ofPyA1in pure water were 7 and 3-fold compared to that in acetonitrile and ethanol. ForPyA2 andPyA3in water-acetonitrile mixture,the FL spectra change was much like that of PyA1. In the mixture of ethanol and cyclohexane,FL emission wavelength was shifted all the time.

|
Download:
|
| Fig. 1. Fluorescence spectra ofPyA13andPyA12in solvent mixtures. ForPyA11in water-acetonitrile and water-ethanol mixtures,water was gradually added. ForPyA12and PyA13in water-acetonitrile,water fraction was increased; in cyclohexane-ethanol,cyclohexane fraction was increased. | |
Interestingly,all the PyA compounds were sensitive to pH changes. The excitation wavelengths ofPyA1-3were 440,460, 465 nm and the emission were 556,560 and 567 nm,respectively. The PyA compounds exhibited light color and quite bright fluorescence emission in pure water solution (pH 7),which can be observed by naked eyes. In pH 2 buffer solution (10 μmol/L PBS),fluorescence almost disappeared and the appearance color of the solutions changed from light green to red. The images were shown in Fig. S2 (Supporting information).

|
Download:
|
| Fig. 2. Fluorescent emission spectra of PyA compounds (10μmol/L) in the presence of different PBS buffer (10 μmol/L,pH 2-7). | |
When the pH value of the solution increased from 2 to 7,the appearance colors of the solutions changed from red to light green. The fluorescence ofPyAcompounds were switched on and enhanced with increasing pH values (Fig. 2). FL intensity changed from 5.3 to 632.0,3.8 to 759.8 and 4.7 to 446.8 for PyA1,PyA2 andPyA3showing 120,200 and 95-fold enhancement,respectively. Among them,PyA2showed the best linearity to pH gradient (Fig. S3 in Supporting information). In the molecular structure ofPyAs,protonation of nitrogen atom on pyridium unit would affect the electron distribution and change the D-π-A structure. At pH 2,fluorescence was quenched. With increasing pH values,they were gradually deprotonated and fluorescence was switched on.
|
Download:
|
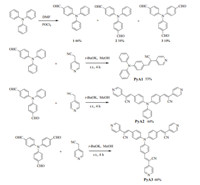
| Fig. 3. (a) Absorption spectra changes of PyA1 (10μmol/L) in water,H+ (100μmol/L) and OH- (100μmol/L). (b) Fluorescence emission of PyA (10μmol/L) in water,H+ (100μmol/L) and OH- (100μmol/L). (c) Acid-base cycles of PyA compounds. (d) Images of color changes ofPyA 1in the presence of acid-base cycles | |
The UV-visible absorption spectra of thesePyAcompounds in different pH values were shown in Fig. S4 (Supporting information). Previous color changes in Fig. S2 indicated that when the pH increased,color of solutions would become much lighter. This could be observed directly from the absorption spectra. The absorption peak tended to be blue shifted with increasing pH values. There was a sudden absorption intensity decrease between pH 2-3,indicating that thesePyAcompounds were sensitive to pH changes. The great fluorescence enhancement and obvious absorption changes endowed thePyAspotential in pH sensing.
|
Download:
|
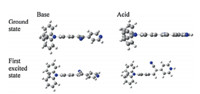
| Fig. 4. Molecular modeling of the interaction between PyAs and proton (employ PyA1as example). | |
The color and fluorescence changes were reversible upon pH cycles. Starting from neutral aqueous conditions,alternative addition of HCl and NaOH solution can adjust the solution to acidic or neutral pH environments. From acid to neutral conditions, absorption intensity tended to decrease (Fig. 3a) and emission was turned on (Fig. 3b). These responses were very fast and can be repeated for several cycles (Fig. 3c). The procedure for preparing samples was shown in Table S1 (Supporting information) [25]. Color changes can be observed by eyes (Fig. 3d,use PyA1 as example). 3.4. Molecular calculation
We went on to investigate the underlying working mechanism of the pH-switched color and fluorescence changes. Generally,these triphenylamines with pyridinium units would work in a protonation/deprotonation mechanism. A reversible chemical reaction of the molecules with OH- /H+ should account for such a phenomenon. We carried out molecular calculation to study the mechanism. As shown in Fig. 4,it is found that after binding with protons,the molecular plane of electron-acceptor group (pyridinium unit) was perpendicular to the electron donor group (TPA) in the first excited state,which caused the formation of TICT (twisted intramolecular charge transfer) excited state and destroyed the D-π-A structure ofPyAsso that the fluorescence was quenched. Upon deprotonation, the D-π-A structure was recovered and fluorescence appeared. So in neutral pH environment,this TICT effect would not take place. 3.5. Agarose gel visualization
We carried out image on agarose gel to check the light emitting properties of these compounds. 1 wt% agarose gels loaded with 10μmol/L of eachPyAcompound were prepared. The gels were immersed in pure water solution and pH 2 buffer solution. Colors and fluorescence images were taken. They showed remarkable difference (Fig. 5). The gel color turned to be red in pH 2 and fluorescence was quenched. In pure water solution,color became light and fluorescence was switched on.

|
Download:
|
| Fig. 5. Colors and fluorescence images of 1 wt% agarose gel loading 10μmol/L of each PyA compound in aqueous and pH 2 buffer solutions. | |
As pH environment is of great importance for normal cell and biomacromolecule functions. It is meaningful to visualize cell pH environment changes. In cell imaging,HeLa cells were first incubated with 10μmol/L of eachPyAcompound for 4 h at 37℃ in PBS buffer solution (10 μmol/L,pH 7),washed with pH 7 PBS buffer three times,and mounted on the microscope stage for imaging. Then,pH 2 PBS was used to replace pH 7 buffer and incubated with cells for 5 min to get the images. As shown in Fig. 6a,Figs. S5a and S6a (Supporting information),PyAswere membrane-permeable and the strong green fluorescence was clearly observed in the cells. In the control experiment,the cells were incubated in pH 2 for 5 min,which contributed to very weak fluorescence (Fig. 6d,Figs. S5d and S6d in Supporting information). The result demonstrated that probe1was capable of imaging pH changes in living cells.

|
Download:
|
| Fig. 6. Confocal laser scanning microscopy analysis of Hela cells. (a) Fluorescence image of Hela cells incubated with PyA1 (10μmol/L) in pH 7 PBS buffer for 4 h. (b) Bright field image of (a). (c) Overlay image of (a) and (b). (d) Fluorescence image of Hela cells incubated with PyA1(10μmol/L) in pH 2 PBS buffer for 5 min. (e) Bright field image of (d). (f) Overlay image of (d) and (e). Images using Ex 440 nm,Em 550 nm. The white bar equals 20mm. | |
In summary,we had designed and synthesized a series of light emitting materials: triphenylamine compoundsPyA1,PyA2and PyA3with different numbers of pyridinium units. These molecules had large Strokes shifts and could emit bright luminescence in the solid state. They were able to sense increasing pH values in a certain range (pH 2-pH 7) by showing different color and emission intensities. It was proposed that the mechanism was related to be the TICT effect through protonation of the pyridinium units. The acid-base switched color and fluorescence intensity changement can be observed under UV irradiation,in water solution and 1% agarose gel. These changes could be repeated for several cycles. We were able to employ these compounds for cell pH environment imaging. The PyA compounds showed potential in materials development and biological fields.
AcknowledgmentsThis work was supported by the National Basic Research Program of China (973 Program) (Nos. 2012CB720600,2012CB720603),the National Science Foundation of China (Nos. 91213302,81373256, 21272181),the National Grand Program on Key Infectious Disease (No. 2012ZX10003002-014),the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT1030),and by the Fundamental Research Funds for the Central Universities.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.11.029.
| [1] | N. Leclerc, S. Sanaur, L. Galmiche, et al., 6-(Arylvinylene)-3-bromopyridine derivatives as lego building blocks for liquid crystal, nonlinear optical, and blue light emitting chromophores, Chem. Mater. 3 (2005) 502-513. |
| [2] | J. Morin, N. Drolet, Y. Tao, M. Leclerc, Syntheses and characterization of electroactive and photoactive 2,7-carbazolenevinylene-based conjugated oligomers and polymers, Chem. Mater. 23 (2004) 4619-4626. |
| [3] | M.S. Wong, Z.H. Li, Y. Tao, M. D'Iorio, Synthesis and functional properties of donor-acceptor π-conjugated oligomers, Chem. Mater 5 (2003) 1198-1203. |
| [4] | W. Zhang, Z. He, Y. Wang, et al., Non-doped red or blue electroluminescent materials based on fluorenyl-triarylamines with fumaronitrile or fluorene bridge, Thin. Solid Films 7 (2012) 2794-2799. |
| [5] | B. An, S. Kwon, S. Jung, S.Y. Park, Enhanced emission and its switching in fluorescent organic nanoparticles, J. Am. Chem. Soc. 48 (2002) 14410-14415. |
| [6] | J. Luo, Z. Xie, J.W. Lam, et al., Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole, Chem. Commun. 18 (2001) 1740-1741. |
| [7] | Y. Liu, X. Tao, F. Wang, et al., Aggregation-induced emissions of fluorenonearylamine derivatives: a new kind of materials for nondoped red organic lightemitting diodes, J. Phys. Chem. C 10 (2008) 3975-3981. |
| [8] | D.U. Kim, S.H. Paik, S. Kim, T. Tsutsui, Design and synthesis of a novel red electroluminescent dye, Synth. Met. 1 (2001) 43-46. |
| [9] | C.L. Chiang, M.F. Wu, D.C. Dai, et al., Red-emitting fluorenes as efficient emitting hosts for non-doped, organic red-light-emitting diodes, Adv. Funct. Mater. 15 (2005) 231-238. |
| [10] | L. Chen, Y. Cui, X. Mei, et al., Synthesis and characterization of triphenylaminosubstituted chromophores for nonlinear optical applications, Dyes Pigments 3 (2007) 293-298. |
| [11] | B. Li, Q. Li, B. Liu, et al., The synthesis and photoluminescence characteristics of novel α,β-diarylacrylonitrile derivatives containing both a biphenyl group and a triphenylamine unit, Dyes Pigments 3 (2011) 301-306. |
| [12] | C. Allain, F. Schmidt, R. Lartia, et al., Vinyl-pyridinium triphenylamines: novel farred emitters with high photostability and two-photon absorption properties for staining DNA, ChemBioChem 4 (2007) 424-433. |
| [13] | T.C. Lin, W.L. Lin, C.M. Wang, C.W. Fu, Synthesis and characterization of highly soluble two-photon-absorbing chromophores with multi-branched and dendritic architectures, Eur. J. Org. Chem. 5 (2011) 912-921. |
| [14] | B. Wang, Y. Wang, J. Hua, et al., Starburst triarylamine donor-acceptor-donor quadrupolar derivatives based on cyano-substituted diphenylaminestyrylbenzene: tunable aggregation-induced emission colors and large two-photon absorption cross sections, Chem. Eur. J. 9 (2011) 2647-2655. |
| [15] | S.J.K. Pond, M. Rumi, M.D. Levin, et al., One-and two-photon spectroscopy of donor-acceptor-donor distyrylbenzene derivatives: effect of cyano substitution and distortion from planarity, J. Phys. Chem. A 47 (2002) 11470-11480. |
| [16] | K. Panthi, R.M. Adhikari, T.H. Kinstle, Aromatic fumaronitrile core-based donor-linker-acceptor-linker-donor (D-p-A-p-D) compounds: synthesis and photophysical properties, J. Phys. Chem. A 13 (2010) 4542-4549. |
| [17] | R. Huang, S. Yan, X. Zheng, et al., Development of a pH-activatable fluorescent probe and its application for visualizing cellular pH change, Analyst 19 (2012) 4418-4420. |
| [18] | F. Galindo, M.I. Burguete, L. Vigara, et al., Synthetic macrocyclic peptidomimetics as tunable pH probes for the fluorescence imaging of acidic organelles in live cells, Angew. Chem. Int. Ed. Engl. 40 (2005) 6504-6508. |
| [19] | H. Lu, B. Xu, Y. Dong, et al., Novel fluorescent pH sensors and a biological probe based on anthracene derivatives with aggregation-induced emission characteristics, Langmuir 9 (2010) 6838-6844. |
| [20] | D. Cui, X. Qian, F. Liu, R. Zhang, Novel fluorescent pH sensors based on intramolecular hydrogen bonding ability of naphthalimide, Org. Lett. 16 (2004) 2757-2760. |
| [21] | B. Tang, X. Liu, K. Xu, et al., A dual near-infrared pH fluorescent probe and its application in imaging of HepG2 cells, Chem. Commun. 36 (2007) 3726-3728. |
| [22] | T. Mallegol, S. Gmouth, M. Meziane, et al., Practical and efficient synthesis of tris(4-formylphenyl)amine, a key building block in materials chemistry, Synthesis 11 (2005) 1771-1774. |
| [23] | H. Lai, Y.J. Xiao, S.Y. Yan, et al., Symmetric cyanovinyl-pyridinium triphenylamine: a novel fluorescent switch-on probe for an antiparallel G-quadruplex, Analyst 139 (2014) 1834-1838. |
| [24] | G.W. Trucks, M.J. Frisch, H.B. Schlegel, et al., Gaussian 09, Revision A 02 ed., Gaussian Inc., Wallingford, CT, 2009. |
| [25] | S.J. Chen, J.Z. Liu, Y. Liu, et al., An AIE-active hemicyanine fluorogen with stimuliresponsive red/blue emission: extending the pH sensing range by ""switch + -knob"" effect, Chem. Sci. 3 (2012) 1804-1809." |