b Shanghai Key Laboratory of Chemical Biology, Institute of Pesticides & Pharmaceuticals, East China University of Science and Technology, Shanghai 200237, China
In the past decade,the environmental protection strategies have led to drastic restrictions concerning sulfur compound content levels in produced fuels because such compounds after gasoil combustion emit SOxand sulfate particulate matter,which are responsible for acid rain,global warming effects,or air pollution [1]. Recently,the application of ionic liquids (ILs) for desulfurization in the research community worldwide has attracted much attention owing to their unique feathers such as negligible vapour pressure,the ability to dissolve a wide range of organic and inorganic compounds,and tailorability [2]. The electrostatic interactions between the cations of ionic liquids and the p-system of the aromatic compounds as well as the formation of liquid clathrates allow for a facile restructuring of the ionic liquids while the aromatic compounds are dissolved [3, 4, 5, 6]. Consequently,under ambient conditions,ILs can easily adsorb aromatic sulfur compounds like dibenzothiophene (DBT) as well as BT which are usually difficult to remove by HDS. Therefore,the desulfurization process using ILs could be considered a complementary technology for the HDS process. By now,apart from AlCl3-based [7],CuCl-based [8],and pyridinium-based ILs [9],many types of studied ionic liquids focused on imidazolium cations, which were utilized for organic sulfur as well as organic nitrogen compounds extraction [10, 11, 12, 13, 14, 15].
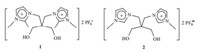
Especially,ILs form extended hydrogen-bond networks between cations and anions,which is a general trend for both the solid and the liquid phase [16]. Accordingly,the imidazolium cations could not interact easily with p-systems in the 1,3-dialkylimidazolium ILs because the H(2) acidic proton was strongly linked to the anion by hydrogen-bonding and aromatic molecules could not cleave this hydrogen-bond [17]. It is inevitable to lead to weak interactions between the imidazolium cations and aromatic molecules,subsequently,causing a high molecular weight which requires large solvent amounts of ILs during desulfurization process. Thus,to solve the bottleneck of this process,for favoringp-interactions between cations of imidazolium ILs and aromatic substrates,the alkylation at position C2 of the imidazolium ring is imperative because it can break apart the H-bond interactions between the cation and the anion [16, 17]. Highlighted by this,a 1,2-dimethylimidazolium IL,2,2-bis((1,2-dimethylimidazolium)methyl) propane-1,3-diol hexafluorophosphate (1) (Scheme 1),which was synthesized according to our initial communications [18, 19],was introduced and used for the desulfurization of fuel oils. The aim of this paper is to attempt to identify and experimentally confirm the nature of potential π-cation interactions between an unsaturated substrate and the imidazolium cation of ILs. A closer look at the interaction with aromatic sulfur compounds is necessary for us to understand how IL cations affect the performances of sulfur removal. In the meantime,it is tried to find an effective way for deep desulfurization of gasoline and diesel fuels.
|
Download:
|
| Scheme 1.The structure of imidazolium ILs1and 2. | |
Imidazolium ILs,2,2-bis((1,2-dimethylimidazolium)methyl)-propane-1,3-diol hexafluorophosphate (1) and 2,2-bis((1-methylimidazolium)methyl)propane-1,3-diol hexafluorophosphate (2) were synthesized by the published procedure [18, 19]. In a typical desulfurization reaction,DBT was dissolved in cyclohexane to simulate the model fuel. The adsorbent desulfurization was carried out in a 50 mL flask containing 2.0 mL of model fuel (sulfur content was 500mg/mL) in the presence of 1. To reach thermodynamic equilibrium,the mixture was vigorously stirred to achieve good contact of both phases and kept overnight in order to settle down. The sulfur content of the organic layer was determined by GC.
After each cycle,adsorbent 1-DBT was collected by filtration and added 10 mL water to precipitate DBT. Filtered to remove DBT, the filtrate was distillated under vacuum to regenerate adsorbent 1,which was reused directly for the next run. 3. Results and discussion
For the beginning of this study,a study to acquire information about the crosssolubility of adsorbent1and the model fuel was investigated because it is considered the key factor in evaluating the applicability of an adsorbent. DBT in cyclohexane was used as a model fuel to study the adsorption capacity of the adsorbent. By analyzing the IL-saturated model fuel sample using HPLC,no IL peak was found. Therefore,the adsorbent1studied here have negligible solubility in the diesel. The solubility of diesel in IL was measured by the gravimetric method [9],was equal to 0.01 wt% in the model fuel corresponding to 10 mL model fuel per mmole of 1, meaning that model fuel is insoluble in the 1.
The results of desulfurization with 1 as an adsorbent for DBT containing model fuels are listed in Table 1. It was found that the sulfur removal was dependent upon temperature or sorption time as well as the molar ratio of 1to aromatic heterocyclic sulfur compound,and in this case,1 shows remarkable ability for sulfur removal at 20℃ in 3 h in terms of 1/1 molar ratio of 1to DBT,more than 77% of DBT have been removed from the model fuel (Table 1, entry 9). However,the sulfur removal ranged from 28.2%,26.9%, 11.9%,to 9.5% with the temperature ranging from 20,30,40,to 50℃ (Table 1,entries 1-4),which implies that,with the increase of temperature,the sorption of the sulfur compound in 1decreases drastically. This observation was interpreted that sulfur compounds adsorbed in 1 could be precipitated by a heating process due to heating IL-sulfur compounds to release the sulfur compounds [13]. The molar ratio of 1 to DBT has a strong influence on the sulfur content after sorption by1.At20℃ and 1 h, 1 gave a sulfur removal of 15.8% and 28.2%,respectively,when the ratio of 1to DBT increased from 1/2 to 1/1 (Table 1,entries 5 and 1). Surprisingly,the presence of a 2/1 or 4/1 molar ration of 1to DBT contributed to lower sulfur removals (Table 1,entries 6 and 7). These worse sulfur removals,to the extent that they reflect the behavior of 1,may be related to accumulation of excessive 1,which inevitably impeded thep-interactions between 1 and the aromatic sulfur compound. In addition,desulfurization maximum reached in 3 h at 20℃ with sulfur removals ranging from 28.2%,40.3%,to 77.7% (Table 1,entries 1,8,and 9),indicating that the reaction time is also an efficient factor to desulfurization under the investigating conditions.
| Table 1 Sulfur removal from model fuel by 1.a |
It was reported that the H(2) acidic proton in the 1,3-dialkylimidazolium cations interacted strongly with anions by hydrogen-bonding,resulting in weak p-systems between cations of ILs and aromatic molecules [17]. However,Gutel’s [5] report showed that aromatic molecule could penetrate the less strongly bonded network and interact with cations when H(2) was replaced by methyl. In the case of 1with H(2) replaced by methyl,it was presumed that the alkylation at position C2 could prevent the formation of hydrogen-bonding with anions and do favor to desulfurization. To clarify this point of view,the desulfurization process was conducted by a 1,3-dialkylimidazolium IL-supported diol (2)(Scheme 1) under the optimized conditions. As we expected,the sulfur removal was in the ranking of 1>2. As shown in Table 1,when the reaction was carried out in the presence of 2featured with one methyl on the imidazolium cation,lower sulfur removal (44.3%) was obtained (Table 1,entry 11). This result is consistent with the before work. Furthermore,for comparison,the capacity of conventional IL [bmim]PF6 fordesulfurizationwasstudiedunderoptimized conditions. The results of the sulfur removal in Table 1 showed that the desulfurization capacityin the investigated ionic liquids was in the order 1>[bmim]PF6,with the sulfur removal being 10.3% for [bmim]PF6 (Table 1,entry 12). In comparison to [bmim]PF6,adsorbent 1 provides,due to its structure and size, more room for the aromatic components in the lattice. Therefore, the capacity of this ionic liquid is higher than the other conventional ionic liquids.
As a remarkable advantage of ionic liquids,the recycle was studied in DBT-containing model fuel. In our experiment,as 1 a solid at room temperature was entirely immiscible with the model fuel. After the reaction,1 was collected by filtration and recycled by precipitating the sulfur compounds using a water dilution process [20, 21, 22],and then reused for the next run. The data shown in Table 1 (entries 13-15) indicated that 1 can be recycled 3 times without a significant decrease in activity.
The experimental results described above suggested that 1 used as an adsorbent for desulfurization maybe more feasible,which could be mainly attributed to the unique structure of 1.Itis reported that the absorption capacity of sulfur compounds by ionic liquids is primarily determined by the size and structure of both cations and anions [9]. As for of 1 as an adsorbent for desulfurization,the presence of methyl at position C2 in the imidazolium ring could successfully prevent hydrogen-bond formation of the H(2) acidic proton with the anions,and consequently increasep-pinteractions between aromatic structures of sulfur compounds and the imidazolium ring systems. Meanwhile,higher polarizable aromaticp-electron densities because of two methyl groups on the imidazolium ring also contributed to the activity. On the other hand,the good desulfurization capacity may derive from more available adsorbing sites in 1. Besides two imidazolium cations,O,O-pinteraction between oxygen atom in the chain and DBT could synergistically gave rise to the high activity of desulfurization [23]. Additionally, larger size of cations of 1could decrease the Coulombic interaction between cations and anions and increasep-interactions [9]. It is obvious that 1 with two 1,2-dimethylimidazolium rings and two OH groups was found to be suitable for desulfurization. Hydrodesulfurization (HDS) is the conventional process for desulfurization in the petroleum refining industry. Nevertheless,owing to stereo hindrance of DBT,higher hydrogen pressure (>4 MPa), higher reaction temperature (>300℃),high-energy cost,and more active catalysts are needed to achieve the very low sulfur content in the HDS process [12]. Compared to HDS reported before, only with 1/1 molar ration of 1to sulfur compound,high sulfur removal (77.7%) could be obtained in the absence of catalysts under the room temperature and pressure. 4. Conclusion
In conclusion,a novel method in which 1,2-dimethylimidazolium IL (1) is used as an adsorbent has been reported. The adsorbent1was found to be effective for the selective removal of aromatic heterocyclic sulfur compound DBT from model fuel at room temperature. The sulfur removal of DBT-containing model fuel can reach 77.7% under the room temperature in 3 h only with 1/1 molar ration of 1to DBT. Moreover,the adsorbent 1 can be recycled 3 times without a significant decrease in activity.
AcknowledgmentsWe acknowledge National Key Technology R and D Program (No. 2011BAE06B05-4),China Postdoctoral Science Foundation (No. 20070410169) for financial support.
| [1] | M. Francisco, A. Arce, A. Soto, Ionic liquids on desulfurization of fuel oils, Fluid Phase Equilib. 294 (2010) 39-48. |
| [2] | L.L. Ban, P. Liu, C.H. Ma, B. Dai, Deep extractive desulfurization of diesel fuels by FeCl3/ionic liquids, Chin. Chem. Lett. 24 (2013) 755-758. |
| [3] | C.G. Hanke, A. Johansson, J.B. Harper, R.M. Lynden-Bell, Why are aromatic compounds more soluble than aliphatic compounds in dimethylimidazolium ionic liquid? A simulation study, Chem. Phys. Lett. 374 (2003) 85-90. |
| [4] | J.D. Holbrey, W.M. Reichert, M. Nieuwenhuyzen, et al., Liquid clathrate formation in ionic liquid-aromatic mixtures, Chem. Commun. (2003) 476-477. |
| [5] | T. Gutel, C.C. Santini, A.A.H. Pádua, et al., Interaction between the pi-system of toluene and the imidazolium ring of ionic liquids: a combined NMRand molecular simulation study, J. Phys. Chem. B 113 (2009) 170-177. |
| [6] | B.M. Su, S. Zhang, Z.C. Zhang, Structural elucidation of thiophene interaction with ionic liquids by multinuclear NMR spectroscopy, J. Phys. Chem. B 108 (2004) 19510-19517. |
| [7] | A. Bösmann, L. Datsevich, A. Jess, et al., Deep desulfurization of diesel fuel by extraction with ionic liquids, Chem. Commun. (2001) 2494-2945. |
| [8] | C. Huang, B. Chen, J. Zhang, Z. Liu, Y. Li, Desulfurization of gasoline by extraction with new ionic liquids, Energy Fuels 18 (2004) 1862-1864. |
| [9] | H. Gao, Y. Li, Y. Wu, et al., Extractive desulfurization of fuel using 3-methylpyridinium-based ionic liquids, Energy Fuels 23 (2009) 2690-2694. |
| [10] | A.R. Hansmeier, G.W. Meindersma, A.B. de Haan, Desulfurization and denitrogenation of gasoline and diesel fuels by means of ionic liquids, Green Chem. 13 (2011) 1907-1913. |
| [11] | A. Revelli, F. Mutelet, J.N. Jaubert, Extraction of benzene or thiophene from n-heptane using ionic liquids. NMR and thermodynamic study, J. Phys. Chem. B 114 (2010) 4600-4608. |
| [12] | L. Lu, S.Cheng, J.Gao, G.Gao,M. He,Deep oxidative desulfurization of fuels catalyzed by ionic liquid in the presence of H2O2, Energy Fuels 21 (2007) 8383-8384. |
| [13] | S. Zhang, Q. Zhang, Z.C. Zhang, Extractive desulfurization and denitrogenation of fuel using of ionic liquids, Ind. Eng. Chem. Prod. Res. 43 (2004) 614-622. |
| [14] | K. Ke-dra-Krolik, F. Mutelet, J.C. MoÏse, J.N. Jaubert, Deep fuels desulfurization and denitrogenation using 1-butyl-3-methylimidazolium trifluoromethanesulfonate, Energy Fuels 25 (2011) 1559-1565. |
| [15] | L. Alonso, A. Arce, M. Francisco, A. Soto, Extraction ability of nitrogen-containing compounds involved in the desulfurization of fuels by using ionic liquids, J. Chem. Eng. Data 55 (2010) 3262-3267. |
| [16] | A. Wulf, K. Fumino, R. Ludwig, Spectroscopic evidence for an enhanced anion-cation interaction from hydrogen bonding in pure imidazolium ionic liquids, Angew. Chem. Int. Ed. 49 (2010) 449-453. |
| [17] | J. Dupont, From molten salts to ionic liquids: a ""nano"" journey, Acc. Chem. Res. 44 (2011) 1223-1231. |
| [18] | Y.Q. Cai, G.H. Song, X. Chen, Investigation on the coordination mode of IL-supported diols used as phosphine-free ligands for palladium catalyzed Heck reaction, Chin. J. Chem. 31 (2013) 1250-1256. |
| [19] | Y.Q. Cai, Y. Liu, G.H. Gao, Synthesis and application of an IL-supported diol as protecting group for aldehydes, Chin. Chem. Lett. 18 (2007) 1205-1208. |
| [20] | H.S. Gao, M.F. Luo, J.M. Xing, et al., Desulfurization of fuel extraction with pyridinium-based ionic liquids, Ind. Eng. Chem. Res. 47 (2008) 8384-8388. |
| [21] | Y. Nie, C.X. Li, A.J. Sun, H. Meng, Z.H. Wang, Extractive desulfurization of gasoline using imidazolium-based phosphoric ionic liquids, Energy Fuels 20 (2006) 2083-2087. |
| [22] | Y. Nie, C.X. Li, Z.H. Wang, Extractive desulfurization of fuel oil using alkylimidazole and its mixture with dialkylphosphate ionic liquids, Ind. Eng. Chem. Res. 46 (2007) 5108-5112. |
| [23] | K.N.M. Daeffler, H.A. Lester, D.A. Dougherty, Functionally important aromatic-aromatic and sulfur-π interactions in the D2 dopamine receptor, J. Am. Chem. Soc. 134 (2012) 14890-14896." |





