b University of Chinese Academy of Sciences, Beijing 100049, China
Prenylated indole alkaloids are a large family of fungal secondary metabolites containing indole/indoline and isoprenoid moieties or structures derived thereof. These alkaloids generally contain a diketopiperazine or a bicyclo[2.2.2]diazaoctane ring as a core structure,and are biogenetically derived from tryptophan,a cyclic amino acid,and one or two isoprene units [1]. Numerous prenylated indole alkaloids including asperparalines,brevianamides,carneamides,chrysogenamides,marcfortines,notoamides, paraherquamides,stephacidins,and versicolamides have been isolated from filamentous fungi,especially from the genera PenicilliumandAspergillus[2]. A broad range of relevant biological activities such as insecticidal,cytotoxic,anthelmintic,and antibacterial properties have been reported,making them attractive targets for chemical synthetic,biosynthetic,and bioactivity studies [3, 4, 5, 6, 7].
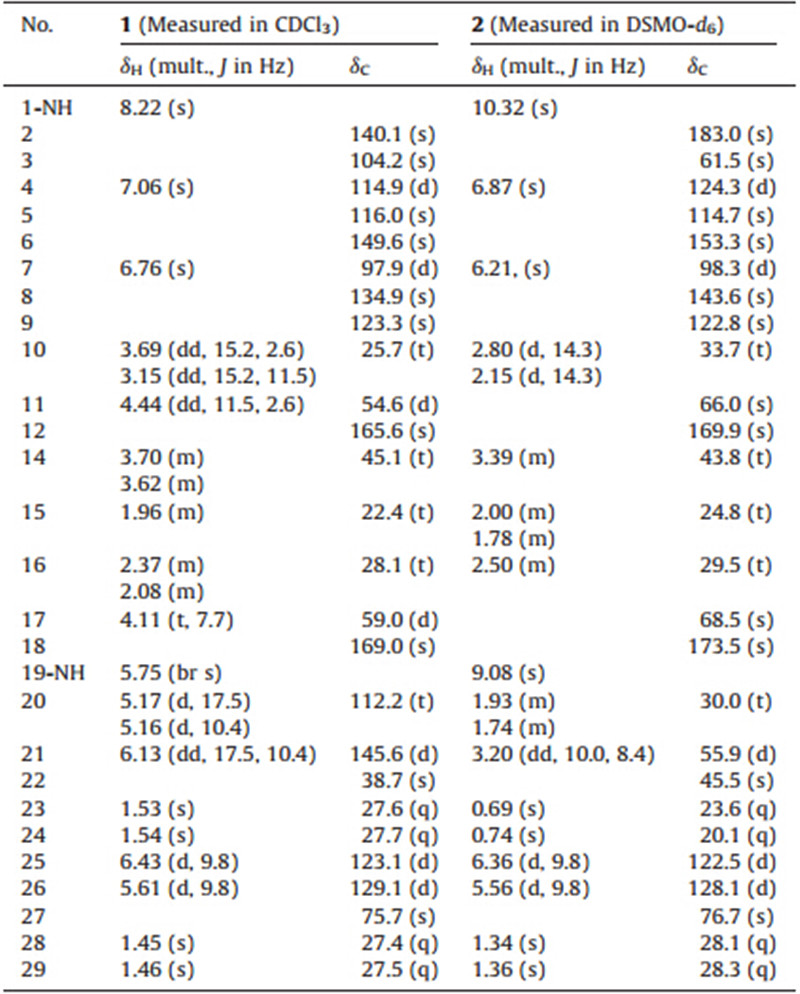
During our ongoing investigation for the bioactive secondary metabolites of marine-derived fungi [8, 9, 10, 11],we have recently reported a new 3H-oxepine-containing diketopiperazine alkaloid (varioxepine A) from the culture of the algal-derived endophytic fungus Paecilomyces variotiiEN-291 [12]. Further work on the fungal extract resulted in the isolation of two new prenylated indole alkaloids,namely,dihydrocarneamide A (1) and isonotoamide B (2) (Fig. 1),from the remaining fractions of the same fungus. It is noteworthy that this is the first time to report the isolation of these alkaloids other than the genera Penicilliumand Aspergillus. Unlike other prenylated indole alkaloids such as asperparalines,notoamides,and versicolamides,compounds 1 and 2 are the rare examples of C-5 prenylation,forming the fused dimethyldihydropyran ring at C-5 and C-6 of the indole ring [3]. Here,we report the isolation,structure identification,and cytotoxic activity of these compounds.
|
Download:
|
| Fig. 1. Structures of dihydrocarneamide A (1) and iso-notoamide B (2). | |
2.1. General experimental procedures
Optical rotations were measured on an Optical Activity AA-55 polarimeter. UV spectra were obtained on a Lengguang Gold S54 photometer. 1D and 2D NMR spectra were recorded on a Bruker Avance 500 MHz spectrometer. Low and high ESI-Mass spectra were determined on a VG Autospec 3000 spectrometer. HPLC analysis was carried out on a Dionex HPLC system (P680 HPLC pump,UVD 340U UV-visible detector) using a C18 column (5mm, 8.0 mm×250 mm,1 mL/min). Commercially available Silica gel (SiO2; 100-200 mesh,200-300 mesh,and GF254) for column chromatography and preparative thin-layer chromatography were produced by Qingdao Haiyang Chemical Group Corporation. RP-18 reverse-phase silica gel (40-63μm) and Sephadex LH-20 were purchased from the Merck Corporation. 2.2. Fungal material
The fungus P. variotiiEN-291 was isolated fromGrateloupia turuturu,a marine red alga collected from the coast of Qingdao, China,in May 2013. The fungus was identified by analysis of its ITS region of the rDNA,as described in our previous report [13]. The sequence data derived from the fungal strain has been deposited at GenBank with the accession number KJ577627. A BLAST search result showed that the sequence was same (100%) to the sequence ofP. variotii(compared with KC311513). The strain is preserved at the Key Laboratory of Experimental Marine Biology,Institute of Oceanology,Chinese Academy of Sciences. 2.3. Fermentation,extraction and isolation
For isolation and identification of new metabolites,the fungal strain was statically fermented in a 1000-mL Erlenmeyer flask containing 300 mL of the PDB medium (potato dextrose broth: 2% mannitol,1% glucose,0.3% peptone,0.5% yeast extract,and seawater added up to 300 mL,pH 6.5-7.0,adjusted with 10% NaOH/flask,60 flasks) at room temperature for 30 days.
The whole fermented cultures (18 L) were filtered to separate the broth from the mycelia. The former was extracted with EtOAc, while the latter was extracted with mixture of 80% acetone anD20% water. Since the TLC and HPLC profiles of the extracts from the broth and mycelia were almost identical,they were combined and concentrated under reduced pressure to give a crude extract (4.3 g) for further separation.
The combined extracts were fractionated by silica gel vacuum liquid chromatography (VLC) using different solvents of increasing polarity from petroleum ether (PE) to methanol (MeOH) to yield 7 fractions (Frs. 1-7) based on TLC analysis. Fr. 7 (0.8 g), eluted with CHCl3-MeOH (10:1),was further purified by column chromatography (CC) on Si gel (CHCl3-MeOH,from 40:1 to 10:1), Lobar LiChroprep RP-18 (MeOH-H2O 3:7-8:2),and Sephadex LH-20 (MeOH)) to afford compounds 1 (67.8 mg) and 2 (11.1 mg).
Dihydrocarneamide A (1): Colorless solid,soluble in CHCl3and MeOH; [a]D20 -33.3 (c0.27,MeOH); UV (MeOH)λmax(logε) 255 (4.36),284 (3.69),291 (3.62),330 (3.44) nm; 1H and 13C NMR,see Table 1; ESI-MSm/z 434 [M+H]+ ,456 [M+Na]+ ; HR-ESIMS m/z 434.2441 [M+H]+ (calcd. for C26H32O3N3 434.2438),456.2257 [M+Na]+ (calcd. for C26H31O3N3Na 456.2258).
| Table 1 1H NMR and 13C NMR data (500 and 125 MHz,respectively) of 1 and2. |
Iso-notoamide B (2): Colorless solid,soluble in MeOH and H2O; [a]D2026.5 (c0.19,MeOH); UV (MeOH)λmax(logε) 235 (4.11),315 (3.45) nm; 1Hand13C NMR,see Table 1; ESI-MSm/z448 [M+H]+ ; HR-ESIMSm/z448.2229 [M+H]+ (calcd. for C26H30O4N3448.2231). 2.4. Absolute configuration of compound1[14, 15]
Dihydrocarneamide A (1,2.8 mg) was hydrolyzed by heating in 6 mol/L HCl (10 mL) at 110℃ for 24 h. The solution was then evaporated to dryness and redissolved in 1 mL of eluting solvent (2 mmol/L CuSO4·5H2OinMeCN-H2O,5:95). Chiral HPLC analysis was carried out using a Phenomenex-Chirex-3126 column at 254 nm with flow rate 1.0 mL/min at 40℃. The HPLC analysis showed that the product of acidic hydrolysis of 1 containedL-Pro,which has the same retention time as that of the standard L-Pro. The result established the chiral center of the proline moiety in the structure of 1 to beS-configuration (Fig. S1 in the Supporting information). 3. Results and discussion
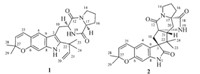
Dihydrocarneamide A (1),obtained as colorless solid,has the molecular formula C26H31N3O3 as established from a prominent pseudomolecular ion peak atm/z434.2441 [M+H]+ in its HRESIMS, implying 13 degrees of unsaturation. In examining the 1H NMR data (Table 1) and HSQC spectra,proton signals in compound 1 were attributable to two exchangeable protons (δH8.22 and 5.75), five aromatic/olefinic methines (δH5.61,6.13,6.43,6.76,and 7.06), one olefinic methylene (δH5.16 and 5.17),four methyl groups (δH 1.45,1.46,1.53,and 1.54) along with two sp3 -hybrided methine and four sp3 -hybrided methylene groups. The 13C NMR spectrum of 1 displayed 26 signals consisting of four methyls,five methylenes (one olefinic),seven methines (five aromatic/olefinic), two amide carbonyls,six sp2 and two sp3 non-protonated carbons as judged by the DEPT spectrum. Among them,signals belong to two carbonyl groups atδC169.0 (C-18) andδC165.6 (C-12) and those at δC 59.0 (C-17) andδC 54.6 (C-11) assignable to two methine carbons were typical of diketopiperazines. Analysis of the 1H- 1H COSY spectrum of1(Fig. 2) established the presence of a proline moiety.
The connection between the different substructures of1was determined by inspection of the HMBC spectrum (Fig. 2). The H-26 signal correlated with the methyl groups CH3-28 and CH3-29 (δC 27.4 and 27.5 ppm,respectively),which was found to constitute a geminal dimethyl moiety,as indicated by their correlation with each other and with an oxygenated nonprotonated carbon atδC75.7 (C-27). Further HMBCs observed indicated a 6,7-disubstituted 2,2-dimethyl-2H-chromone moiety in which a benzene ring,a double bond,a geminal dimethyl moiety,and an oxygen were incorporated. The reverse prenyl unit was determined to be connected with C-2 as evidenced by the observed HMBC correlations from H-21 to C-2. NOE correlation between H-11 and H-17 indicated that the diketopiperazine ring wascis-configured. Acidic hydrolysates of1by chiral HPLC analysis showed the presence of L-proline (Fig. S1). Therefore,the stereogenic centers of 1 were determined as 11S and 17S.

|
Download:
|
| Fig. 2. Key HMBC (arrows) and COSY (bold lines) correlations of compounds 1 and2. | |
Compound 2 was assigned the molecular formula C26H29N3O4 on the basis of HRESIMS data (m/z448.2229 [M+H]+ ),which was supported by the 1H and 13C NMR data (Table 1). Detailed analysis of 1D NMR data as well as 2D NMR correlations indicated that the structure of compound 2 was similar to that of notoamide B [16], which contains the core bicyclo[2.2.2]diazaoctane moiety,aspirooxindole ring system with a pyran group,and an unsubstituted proline ring,except for the coupling patterns in the aromatic region of the 1H NMR spectrum,e.g.,singlet for H-4 in 2vs.doublet (J= 8.5 Hz) for H-4 in notoamide B [16]. Observed HMBC correlations from H-21 to C-11,C-22,and C-23,from exchangeable amide NH proton H-19 to C-11 and C-17,and from H-20 to C-17 and C-18 indicated the presence of the bicyclo[2.2.2]diazaoctane ring,which is generated from the rearrangement of a diketopiperazine ring and an isoprenyl group.
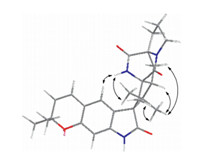
The relative configurations of2were deduced by analysis of NOESY data. Previous studies revealed that the 2-oxindole alkaloid with bicyclo[2.2.2]diazaoctane ring adopted a conformation in which the central five-membered ring is orthogonal to the plane of the oxindole subunit [17]. NOE correlations between H-4,H-10β,H3-24 and 19-NH indicated that H-10b and H3-24 are both on the face of the cyclopentanoid ring that orients them toward H-4,fixing the relative configuration at C-3 as shown (Fig. 3). NOE interactions between H-21,H-10a,H-20a and H3-23 placed the corresponding substituents together on the opposite face of the cyclopentanoid ring. The proposed relative configurations of 2 are in agreement with the relevant features of the relative stereochemistry of versicolamide B [18].
|
Download:
|
| Fig. 3. Key NOE correlations of compound2. | |
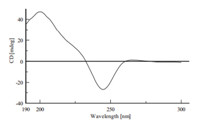
The absolute configurations of 2 were elucidated by CD spectrum (Fig. 4). Williams and co-workers have reported that the Cotton effect at 200-250 nm arising from an n-π* transition of the diketopiperazine amide bonds is diagnostic for the bicyclo[2.2.2]diazaoctane ring system and the Cotton effect at250-350 nm is diagnostic of the configuration atspiro-stereogenic center C-3 [19]. The CD spectrum of 2 correlated to relevant regions of that of (+)-versicolamide B [18],thus the absolute configurations of2was assigned as 3S,11S,17S,and 21R.
|
Download:
|
| Fig. 4. CD spectrum of2in MeOH. | |
Compounds 1 and 2 were assayed for their cytotoxic activities against NCI-H460 (human large cell lung carcinoma cell line). These two compounds showed weak activity with IC50values of 69.3 and 55.9mmol/L,respectively. 4. Conclusion
Prenylated indole alkaloids including asperparalines,brevianamides,notoamides,paraherquamides,stephacidins,and versicolamides,which all contain the core bridging bicyclo[2.2.2]-diazaoctane ring system,are commonly observed natural products mainly from the generaPenicillium and Aspergillus. In the structures of these alkaloids,the prenylation normally occurred at C-7 and then formed a fused dimethyldihydropyran ring with C-6. However,for the carneamides,the dimethyl-substituted pyran ring coupled with the indole moiety at C-5 and C-6,which is rare in this type of alkaloids. In this paper,we described the isolation and identification of two new indolediketopiperazines, dihydrocarneamide A (1) and iso-notoamide B (2) that having the dimethyl-substituted pyran ring coupled with the indole moiety at C-5 and C-6,from the marine-derived endophytic fungusP. variotii EN-291. Biological evaluation indicated that 1 and 2 had weak cytotoxicities against NCI-H460 tumor cells.
AcknowledgmentsFinancial support from the Natural Science Foundation of China (No. 31330009) and from the Ministry of Science and Technology of China (No. 2010CB833802) is gratefully acknowledged.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.11.020
| [1] | J.M. Finefield, H. Kato, T.J. Greshock, et al., Biosynthetic studies of the notoamides: isotopic synthesis of stephacidin A and incorporation into notoamide B and sclerotiamide, Org. Lett. 13 (2011) 3802-3805. |
| [2] | J.M. Finefield, J.C. Frisvad, D.H. Sherman, R.M. Williams, Fungal origins of the bicyclo[2.2.2]diazaoctane ring system of prenylated indole alkaloids, J. Nat. Prod. 75 (2012) 812-833. |
| [3] | O.I. Zhuravleva, S.S. Afiyatullov, V.A. Denisenko, et al., Secondary metabolites from a marine-derived fungus Aspergillus carneus Blochwitz, Phytochemistry 80 (2012) 123-131. |
| [4] | S.X. Cai, Y.P. Luan, X.L. Kong, et al., Isolation and photoinduced conversion of 6-epi-stephacidins from Aspergillus taichungensis, Org. Lett. 15 (2013) 2168-2171. |
| [5] | T.J. Greshock, A.W. Grubbs, S. Tsukamoto, R.M. Williams, A concise, biomimetic total synthesis of stephacidin A and notoamide B, Angew. Chem. Int. Ed. 46 (2007) 2262-2265. |
| [6] | Y. Ding, J.R. de Wet, J. Cavalcoli, et al., Genome-based characterization of two prenylation steps in the assembly of the stephacidin and notoamide anticancer agents in a marine-derived Aspergillus sp., J. Am. Chem. Soc. 132 (2010) 12733-12740. |
| [7] | P.S. Baran, B.D. Hafensteiner, N.B. Ambhaikar, C.A. Guerrero, J.D. Gallagher, Enantioselective total synthesis of avrainvillamide and the stephacidins, J. Am. Chem. Soc. 128 (2006) 8678-8693. |
| [8] | Y. Zhang, X.M. Li, C.Y. Wang, B.G. Wang, A new naphthoquinoneimine derivative from the marine algal-derived endophytic fungus Aspergillus niger EN-13, Chin. Chem. Lett. 18 (2007) 951-953. |
| [9] | C.S. Li, X.M. Li, S.S. Gao, Y.H. Lu, B.G. Wang, Cytotoxic anthranilic acid derivatives from deep sea sediment-derived fungus Penicillium paneum SD-44, Mar. Drugs 11 (2013) 3068-3076. |
| [10] | X. Li, X.M. Li, G.M. Xu, C.S. Li, B.G. Wang, Antioxidant metabolites from marine alga-derived fungus Aspergillus wentii EN-48, Phytochem. Lett. 7 (2014) 120-123. |
| [11] | M.H. Wang, X.M. Li, C.S. Li, N.Y. Ji, B.G. Wang, Secondary metabolites from Penicillium pinophilum SD-272, a marine sediment-derived fungus, Mar. Drugs 11 (2013) 2230-2238. |
| [12] | P. Zhang, A. Mándi, X.M. Li, et al., Varioxepine A, a 3H-oxepine-containing alkaloid with a new oxa-cage from the marine algal-derived endophytic fungus Paecilomyces variotii, Org. Lett. 16 (2014) 4834-4837. |
| [13] | S. Wang, X.M. Li, F. Teuscher, et al., Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata, J. Nat. Prod. 69 (2006) 1622-1625. |
| [14] | Y. Li, K.L. Sun, Y. Wang, et al., A cytotoxic pyrrolidinoindoline diketopiperazine dimer from the algal fungus Eurotium herbariorum HT-2, Chin. Chem. Lett. 24 (2013) 1049-1052. |
| [15] | B.H. Han, H. Gross, D.E. Goeger, S.L. Mooberry, W.G. Gerwick, Aurilides B and C, cancer cell toxins from a Papua New Guinea collection of the marine cyanobacterium Lyngbya majuscula, J. Nat. Prod. 69 (2006) 572-575. |
| [16] | H. Kato, T. Yoshida, T. Tokue, et al., Notoamides A-D: prenylated indole alkaloids isolated from a marine-derived fungus, Aspergillus sp., Angew. Chem. Int. Ed. 46 (2007) 2254-2256. |
| [17] | A.C. Whyte, J.B. Gloer, D.T. Wicklow, P.F. Dowd, Sclerotiamide: a new member of the paraherquamide class with potent antiinsectan activity from the sclerotia of Aspergillus sclerotiorum, J. Nat. Prod. 59 (1996) 1093-1095. |
| [18] | T.J. Greshock, A.W. Grubbs, P. Jiao, et al., Isolation, structure elucidation, and biomimetic total synthesis of versicolamide B, and the isolation of antipodal (-)-stephacidin A and (+)-notoamide B from Aspergillus versicolor NRRL 35600, Angew. Chem. Int. Ed. 47 (2008) 3573-3577. |
| [19] | R.M. Williams, E. Kwast, H. Coffman, T. Glinka, Remarkable, enantio-divergent biogenesis of brevianamide A and B, J. Am. Chem. Soc. 111 (1989) 3064-3065." |