b Department of Chemistry, Osmania University, Hyderabad 500 007, India
The development of cleaner technologies is a major subject in green chemistry [1]. Among the several aspects of green chemistry, the reduction or replacement of volatile organic solvents from the reaction medium is of greatest concern. Ionic liquids (ILs) have received considerable interest as eco-friendly solvents,catalysts and reagents in the context of green synthesis because of their unique properties such as low volatility,non-flammability,high thermal stability,negligible vapour pressure and ability to dissolve a wide range of materials [2].
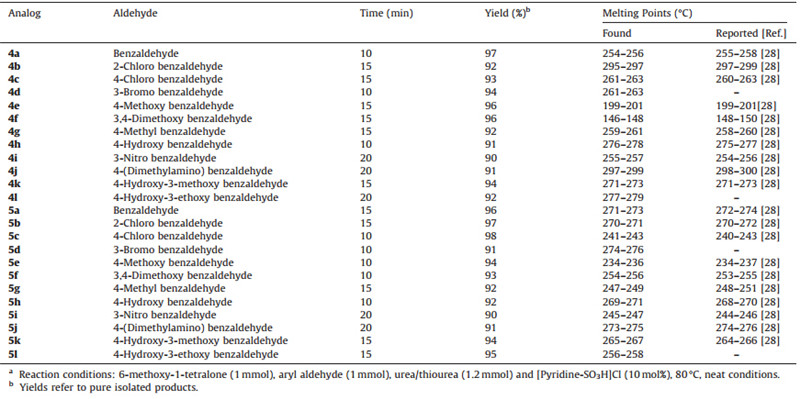
Dihydropyrimidinones (DHPMs) were found to possess several biological activities such as antimicrobial,antiviral,antimalarial, anticancer,antihypertensive,anti-inflammatory,calcium channel modulators,mitotic kinesin inhibitors,a1A-antagonists and neuropeptide Y(NPY) antagonists [3, 4, 5, 6, 7]. Several biologically active marine alkaloids were also found to contain the dihydropy rimidinone-5-carboxylate core. Most notable among them are batzelladine alkaloids,which have been found to be potent HIVgp-120-CD4 inhibitors [8]. Some of the biologically active dihydropyrimidine derivatives have shown in Fig. 1.
|
Download:
|
| Fig. 1. StructuresSome of the biologically potent DHPMs. | |
3,4-Dihydropyrimidin-2(1H)-ones was first reported by Pietro Biginelli in 1893viaa three component condensation of benzaldehyde,β-ketoester and urea under strongly acidic conditions [9]. However,it often requires harsh reaction conditions, longer reaction time and affords low yields,particularly when substituted aromatic and aliphatic aldehydes are employed. To avoid these limitations,several methods were reported utilizing different catalytic systems such as CaF2[10],PPh3[11],BF3(OEt)2 [12],FeCl3·6H2O [13],InCl3 [14],YbCl3 [15],BiCl3 [16], Fe(OTs)3·6H2O [17],Ce(C12H25SO3)3 [18],HCl/EtOH [19],chloroacetic acid [20],1-carboxymethyl-3-methylimidazolium hydrogen sulfate [21],silica sulfuric acid [22],cellulose sulfuric acid [23], silica-bondedN-propyl sulfamic acid [24],potassium phthalimide [25] and acidic ionic liquids [26]. Nevertheless,many of the reported methods suffer from one or several drawbacks such as longer reaction time,low yield of the products,complex isolation procedure,harsh reaction conditions and use of large amount of expensive reagents. Therefore,the present communication aims to introduce a mild,efficient and eco-friendly protocol for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones and thiones in the presence of acidic ionic liquid,1-sulfopyridinium chloride [pyridine-SO3H]Cl under solvent-free conditions. 2. Experimental
All the solvents and chemicals were purchased from Aldrich/ Merck and used without further purifications. Melting points were obtained in open capillaries using Stuart SMP30 melting point apparatus and are uncorrected. The progress of the reactions as well as purity of the compounds was monitored by thin layer chromatography,and the developed chromatogram was visualized with UV light and iodine vapors. IR spectra were recorded on Perkin-Elmer 100S spectrophotometer using KBr disk. 1H NMR spectra were recorded on Bruker-400 MHz spectrometer using TMS as an internal standard. The C,H and N analyses of the compounds were done on a Carlo Erba EA1108 analytical unit and mass spectra were recorded on a JEOL JMSD-300 spectrometer.
General procedure for the synthesis of fused 3,4-dihydropyrimidine-2(1H)-ones (4a-l)andthiones(5a-l): Ionic liquid [pyridine-SO3H]Cl (10 mol%) was added to a mixture of 6-methoxy-1-tetralone (1,1 mmol),aromatic aldehydes (2a-l, 1 mmol) and urea/thiourea (3a/b,1.2mmol),andheatedat 80℃ under solvent-free conditions for an appropriate time. After completion of the reaction (monitored by TLC),the product was extracted by the warm ethyl acetate and purified by recrystallization from ethanol. The recovered catalyst was washed with ethyl acetate,dried under vacuum at 90℃ for about 3 h and reused for subsequent reaction.
4-(3-Bromophenyl)-8-methoxy-3,4,5,6-tetrahydrobenzo[h]-quinazolin-2(1H)-one (4d): Pale yellow solid; IR (KBr,cm-1 ):ymax 3237 (NH),1681 (C=0),1178 (C-O-C),764 (C-Br); 1H NMR (400 MHz,DMSO-d6.):δ1.71-1.79 (m,1H),2.07-2.15 (m,1H), 2.54-2.60 (m,1H),2.65-2.72 (m,1H),3.73 (s,3H),4.93 (s,1H),6.76 (t,2H,J= 6.4 Hz),7.29-7.34 (m,3H),7.47-7.53 (m,3H),8.54 (s,1H); MS (ESI):m/z385 [M+1]+ ; Anal. calcd. for C19H17BrN2O2:C, 59.23; H,4.45; N,7.27. Found: C,59.38; H,4.36; N,7.12.
4-(3-Ethoxy-4-hydroxyphenyl)-8-methoxy-3,4,5,6-tetrahydrobenzo[h]quinazolin-2(1H)-one (4l): Pale yellow solid; IR (KBr, cm-1 ):ymax3359 (OH),3180 (NH),1686 (C=0),1172 (C-O-C); 1H NMR (400 MHz,DMSO-d6.):δ1.30 (t,3H,J= 6.8 Hz),1.73-1.81 (m, 1H),2.02-2.10 (1H),2.54-2.69 (m,2H),3.73 (s,3H),3.94-3.99 (m, 2H),4.78 (s,1H),6.68-6.75 (m,4H),6.84 (d,1H,J= 6.4 Hz),7.07 (s, 1H),7.49 (d,1H,J= 9.6 Hz),8.39 (s,1H),8.86 (s,1H); MS (ESI):m/z 367 [M+1]+ ; Anal. calcd. for C21H22N2O4: C,68.84; H,6.05; N, 7.65. Found: C,68.95; H,5.91; N,7.35.
4-(3-Bromophenyl)-8-methoxy-3,4,5,6-tetrahydrobenzo[h]-quinazoline-2(1H)-thione (5d): Pale yellow solid; IR (KBr,cm-1 ): ymax3179 (NH),1251 (C=5),1185 (C-O-C),741(C-Br); 1H NMR (400 MHz,DMSO-d6.):δ1.79-1.87 (m,1H),2.14-2.21 (m,1H), 2.56-2.61 (m,1H),2.67-2.73 (m,1H),3.75 (s,3H),4.96 (s,1H),6.78 (s,2H),7.30-7.38 (m,2H),7.50 (t,2H,J= 7.6 Hz),7.63 (d,1H, J= 9.2 Hz),9.08 (s,1H),9.78 (s,1H); MS (ESI):m/z402 [M+1]+ ; Anal. calcd. for C19H17N2OS: C,56.86; H,4.27; N,6.98. Found: C,56.75; H, 4.41; N,6.79.
4-(3-Ethoxy-4-hydroxyphenyl)-8-methoxy-3,4,5,6-tetrahydrobenzo[h]quinazolin-2(1H)-thione (5l): Pale yellow solid; IR (KBr,cm-1 ):ymax3354 (OH),3183 (NH),1262 (C=5),1171 (C-O- C); 1H NMR (400 MHz,DMSO-d6.):δ1.31 (t,3H,J= 6.8 Hz),1.81- 1.88 (m,1H),2.08-2.16 (m,1H),2.54-2.71 (m,2H),3.74 (s,3H), 3.94-4.00 (m,2H),4.79 (s,1H),6.68 (t,1H,J= 8.4 Hz),6.76 (d,3H, J= 7.6 Hz),6.84 (s,1H),7.61 (d,1H,J= 9.2 Hz),8.92 (s,1H),8.95 (s,1H),9.61 (s,1H); MS (ESI): m/z 383 [M+1]+ ; Anal. calcd. For C21H22N2O3S: C,65.95; H,5.80; N,7.32. Found: C,65.82; H, 5.89; N,7.09. 3. Results and discussion
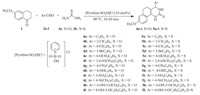
Fused 3,4-dihydropyrimidin-2(1H)-ones (4a-l) and thiones (5a-l) were obtained by the one-pot three component condensation of 6-methoxy-1-tetralone (1),aromatic aldehyde (2a-l) and urea (3a)/thiourea (3b) utilizing acidic ionic liquid,[pyridineSO3H]Cl as a catalyst under solvent-free conditions in excellent yields. The schematic representation has shown in Scheme 1. The ionic liquid,[pyridine-SO3H]Cl has prepared according to the literature procedure [27].
|
Download:
|
| Scheme 1.1-Sulfopyridinium chloride catalyzed synthesis of fused 3,4-dihydropyrimidine-2(1H)-ones and thiones. | |
To evaluate the feasibility of 1-sulfopyridinium chloride,a modal reaction involving benzaldehyde (2a),6-methoxy-1-tetralone (1) and thiourea (3b) with a building block ratio of 1:1:1.2 was carried out at different temperatures (r.t.,40,80 and 120℃) in the absence as well as in the presence of different amount of catalyst (5- 15 mol%) under solvent-free conditions (Table 1). At room temperature,with and without catalyst product (5a) formation was not observed. In absence of catalyst,as the temperature increases the yield of the product has slightly increased and observed maximum yield (10%) at 80℃. In the presence of catalyst, maximum yield (96%) was observed at 80℃ with 10 mol% of the catalyst. Further increment of temperature and amount of catalyst has not shown any affect on product yield and reaction time. The same reaction was also carried out with 10 mol% of catalyst in different solvents like ethanol,acetic acid and water under reflux conditions. But the yield of the product (5a) obtained was lower compare to the reaction under solver-free conditions (Table 1).
| Table 1 Optimizing the reaction conditions.a |
At these optimistic conditions (10 mol% of catalyst,solvent-free conditions,80℃),a series of 3,4-dihydropyrimidine-2(1H)-ones (4a-l) and thiones (5a-l) were obtained by varying the aromatic aldehyde and urea/thiourea with excellent yields in shorter reaction time (Table 2). After completion of the reaction the catalyst was recovered from the reaction mixture by washing with warm ethyl acetate,dried under vacuum at 90℃ and reused for subsequent reactions. For example,the reaction of 6-methoxy-1-tetralone (1),benzaldehyde (2a) and thiourea (3b) gave the corresponding 3,4-dihydropyrimidin-2(1H)-thione (5a) in 96%, 94%,91%,90% and 90% yields over additional five cycles.
| Table 2 1-Sulfopyridinium chloride catalyzed synthesis of fused 3,4-dihydropyrimidine-2(1H)-ones and thiones under solvent-free conditions.a |
In conclusion,we have developed a facile route for the synthesis of fused 3,4-dihydropyrimidine-2(1H)-ones and thiones in the presence of ionic liquid [pyridine-SO3H]Cl under solvent-free conditions. This method has several advantages such as simple work-up procedure,involving short reaction time,high yields of the product formation,eco-friendly and reusability of catalyst.
AcknowledgmentsWe would like to thank the Director,National Institute of Technology-Warangal for providing research facilities. One of the author’s (RV) thanks to CSIR-UGC New Delhi,India for providing research fellowships.
| [1] | W. Zhang, B.W. Cue, Green Techniques for Organic Synthesis and Medicinal Chemistry, Wiley, Chichester, 2012. |
| [2] | A.M. Inamuddin, Green Solvents II: Properties and Applications in Chemistry, Springer, London, 2012. |
| [3] | A. Agarwal, K. Srivastava, S.K. Puri, P.M.S. Chauhan, Antimalarial activity and synthesis of new trisubstituted pyrimidines, Bioorg. Med. Chem. Lett. 15 (2005) 3130-3132. |
| [4] | H.T. Rajesh, H.R. Atish, D.H. Girish, et al., The novel 3,4-dihydropyrimidin-2(1H)-one urea derivatives of N-aryl urea: synthesis, anti-inflammatory, antibacterial and antifungal activity evaluation, Bioorg. Med. Chem. Lett. 21 (2011) 4648-4651. |
| [5] | S.W. Fewell, C.M. Smith, M.A. Lyon, et al., Small molecule modulators of endogenous and co-chaperone-stimulated Hsp70 ATPase activity, Biol. Chem. 279 (2004) 51131-51140. |
| [6] | C.O. Kappe, Biologically active dihydropyrimidones of the Biginelli-type -a literature survey, Eur. J. Med. Chem. 35 (2000) 1043-1052. |
| [7] | K.S. Atwal, B.N. Swanson, S.E. Unger, et al., Dihydropyrimidine calcium channel blockers. 3. 3-Carbamoyl-4-aryl-1,2,3,4-tetrahydro-6-methyl-5-pyrimidinecarboxylic acid esters as orally effective antihypertensive agents, J. Med. Chem. 34 (1991) 806-811. |
| [8] | A.D. Patil, N.V. Kumar, W.C. Kokke, et al., Novel alkaloids from the sponge Batzella: inhibitors of HIV gp120-human CD4 binding, J. Org. Chem. 60 (1995) 1182-1188. |
| [9] | P. Biginelli, Aldehyde-urea derivatives of aceto-and oxaloacetic acids, Gazz. Chim. Ital. 23 (1893) 360-413. |
| [10] | S. Chitra, K. Pandiarajan, Calcium fluoride: an efficient and reusable catalyst for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones and their corresponding 2(1H)thione: an improved high yielding protocol for the Biginelli reaction, Tetrahedron Lett. 50 (2009) 2222-2224. |
| [11] | A. Debache, M. Amimour, A. Belfaitah, et al., A one-pot Biginelli synthesis of 3,4-dihydropyrimidin-2-(1H)-ones/thiones catalyzed by triphenylphosphine as Lewis base, Tetrahedron Lett. 49 (2008) 6119-6121. |
| [12] | E.H. Hu, D.R. Sidler, U.H. Dolling, Unprecedented catalytic three component one-pot condensation reaction: an efficient synthesis of 5-alkoxycarbonyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones, J. Org. Chem. 63 (1998) 3454-3457. |
| [13] | J. Lu, H. Ma, Iron(III)-catalyzed synthesis of dihydropyrimidinones, improved conditions for the Biginelli reaction, Synlett (2000) 63-64. |
| [14] | C.R. Brindaban, A. Hajra, U. Jana, Indium(III) chloride-catalyzed one-pot synthesis of dihydropyrimidinones by a three-component coupling of 1,3-dicarbonyl compounds, aldehydes, and urea: an improved procedure for the Biginelli reaction, J. Org. Chem. 65 (2000) 6270-6272. |
| [15] | H. Zhang, Z. Zhou, Z. Yao, et al., Efficient synthesis of pyrimidinone derivatives by ytterbium chloride catalyzed Biginelli-type reaction under solvent-free conditions, Tetrahedron Lett. 50 (2009) 1622-1624. |
| [16] | K. Ramalinga, P. Vijayalakshmi, T.N.B. Kaimal, Bismuth(III)-catalyzed synthesis of dihydropyrimidinones: improved protocol conditions for the Biginelli reaction, Synlett 6 (2001) 863-865. |
| [17] | J.T. Starcevich, T.J. Laughlin, R.S. Mohan, Iron(III) tosylate catalyzed synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones via the Biginelli reaction, Tetrahedron Lett. 54 (2013) 983-985. |
| [18] | Y. Qiu, H. Sun, Z. Ma, et al., Efficient, stable, and reusable Lewis acid-surfactantcombined catalyst: one-pot Biginelli and solvent-free esterification reactions, J. Mol. Catal. A: Chem. 392 (2014) 76-82. |
| [19] | J. Svetlik, V. Kettmann, The chameleon-like behaviour of 3-amino-1,2,4-triazole in the Biginelli reaction: unexpected formation of a novel spiroheterocyclic system, Tetrahedron Lett. 52 (2011) 1062-1066. |
| [20] | I. Couto, I. Tellitu, E. Dominguez, Searching for a direct preparation of dihydropyrimidine-5-carboxamides under Biginelli reaction conditions, Arkivoc II (2011) 115-126. |
| [21] | F. Makaev, E. Styngach, V. Shargarovskii, et al., Imidazolium salts with a free carboxy group as new catalysts of the Biginelli reaction, Russ. J. Org. Chem. 46 (2010) 616-617. |
| [22] | W.Y. Chen, S.D. Qin, J.R. Jin, Efficient Biginelli reaction catalyzed by sulfamic acid or silica sulfuric acid under solvent-free conditions, Syn. Commun. 37 (2007) 47-52. |
| [23] | P.N. Reddy, Y.T. Reddy, M.N. Reddy, et al., Cellulose sulfuric acid: an efficient biodegradable and recyclable solid acid catalyst for the one-pot synthesis of 3,4-dihydropyrimidine-2(1H)-ones, Syn. Commun. 39 (2009) 1257-1263. |
| [24] | S.R. Jetti, A. Bhatewara, T. Kadre, et al., Silica-bonded N-propyl sulfamic acid as an efficient recyclable catalyst for the synthesis of 3,4-dihydropyrimidin-2-(1H)-ones/thiones under heterogeneous conditions, Chin. Chem. Lett. 25 (2014) 469-473. |
| [25] | H. Kiyani, M. Ghiasi, Potassium phthalimide: an efficient and green organocatalyst for the synthesis of 4-aryl-7-(arylmethylene)-3,4,6,7-tetrahydro-1H-cyclopenta[d]pyrimidin-2(5H)-ones/thionesunder solvent-free conditions,Chin.Chem.Lett. 25 (2014) 313-316. |
| [26] | J. Gui, D. Liu, C. Wang, et al., One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones catalyzed by acidic ionic liquids under solvent-free condition, Synth. Commun. 39 (2009) 3436-3443. |
| [27] | A.R.M. Zare, M.A. Zolfigol, M. Zarei, et al., Design, characterization and application of new ionic liquid 1-sulfopyridinium chloride as an efficient catalyst for tandem Knoevenagel-Michael reaction of 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one with aldehydes, Appl. Catal. A 467 (2013) 61-68. |
| [28] | B. Janardhan, B. Rajitha, A. Peter, Crooks poly(4-vinylpyridinium) hydrogen sulfate: an efficient and recyclable Bronsted acid catalyst for the synthesis of fused 3,4-dihydropyrimidin-2(1H)-ones and thiones, J. Saudi Chem. Soc. (2013), http://dx.doi.org/10.1016/j.jscs.2012.10.007." |