b Materials and Chemical Engineering, Hai Nan University, Hainan 570228, China
Ino(Rg)anic nanomaterials with hollow and mesoporous superstructures find numerous technological applications where morphologies are known to influence functionality [1]. Gas sensors [2],catalysts [3],drug delivery carriers [4],and light absorption building blocks [5, 6] are just a few noteworthy examples. In general,morphology with a la(Rg)e specific surface area and high porosity is usually beneficial for the catalytic,gas-sensing and photovoltaic applications due to the likelihood to enhance surface reactions. In this respect,the active search of unusual morphology is currently the subject of intensive research in the world of nanomaterials. Zinc oxide,as an exceptionally important versatile metal oxide,is an n-type semiconductor with a direct wide band gap (3.37 eV) and a la(Rg)e excitation binding ene(Rg)y (60 meV),and has been considered an ideal material for field effect transistors [7], solar cells [8],luminescent materials [9],photocatalysts [10], chemical sensors [11],and so on. To meet the demand for particular applications,various distinct ZnO structures have been synthesized,including rods [12],wires [13],and tubes (1D) [14], rings and cups (2D) [15],etc. Recently,ZnO hollow structures have attracted a great deal of attention because of their low density,high surface area,and efficient surface permeability. Owing to such characteristics,ZnO hollow structures can be employed in various applications including drug delivery,catalysis,chemical storage, microcapsule reactors,sensors,etc. So far,hollow ZnO fibers have been extensively fabricated using o(Rg)anic fiber as templates [16, 17]. Hollow ZnO microspheres have also been synthesized using thermolysis [18],template [19, 20, 21] or template-free hydrothermal methods [22, 23]. Chenet al. reported well-defined ZnO hollow microspheres synthesized by a facile solvothermal process [24]. Hierarchical,ZnO porous,hollow spheres with controllable shell numbers were synthesized by different temperature gradient processes using a simplified hard template method [25]. Among these fabrication methods,a template-free hydrothermal method is preferably used in the fabrication of hollow ZnO microspheres by using physical phenomena,e.g..,oriented attachment [26],Ostwald ripening [27, 28, 29],Kirkendall effect [30, 31]. Also,Wanget al. [32] reviewed the recent advances in synthesis of hollow structures and discussed the methodologies for controlling the number of multi-shelled hollow spheres. Though the above reports carefully investigated the influence of reaction temperature and time on the morphology of the ‘‘as-synthesized’’ samples, the influence of the chemical composition on the morphologies of mesoporous hollow spheres still remains unclear. Moreover, further efforts are still necessary to precisely control the porosity of the hollow sphere structure.
Herein,we have synthesized mesoporous hollow ZnO microspheres under a controllable mannerviaa sovolthermal method, using glycerol and zinc acetate dihydrate as the starting materials. The influence of the reagent ratio on the morphologies of the microspheres was investigated in detail. In particular,the mesoporous shell structure of the hollow microspheres resulted in unusual UV emission and adsorption similarly found in blackbody phenomena. 2. Experimental
All reagents used in the experiment are of analytical grade without additional purification. All ZnO microspheres were synthesized using a hydrothermal method. Zinc acetate dihydrate (Zn(CH3COO)2·2H2O) (1-3 g) was first dissolved in 10 ml of distilled water,and then was dissolved in 60 mL of glycerol (C3H8O3) under mechanically stirring for 1 h. The solution was then transferred to an autoclave,which was then heated to 200℃ for a determined time to produce precipitation. The ZnO products was obtained by centrifuging,washing with distilled water and ethanol to remove unnecessary ions,and dried at 60℃ for 24 h in air. The samples synthesized in the reaction system with the weight ratio (Rw) of 1:10:75,2:10:75 and 3:10:75 were marked as G1,G2 and G3,respectively,whereRwwas the defined weight ratio of Zn(CH3COO)2·2H2O:H2O:C3H8O3.
Crystalline analysis was conducted using a DX-2000 X-ray diffractometer with CuKaradiation (λ=0.1542 nm) operated at 40 kV and 20 mA. The data were collected at a scanning rate of 0.1 deg s-1 for 2θ values. Morphologies of the samples were observed using a 1530VP model field emission scanning electron microscope. Room temperature photoluminescence (PL) spectra of the products were recorded on F-7000 Fluorescence spectrometer using the 325 nm Xe laser light as an excitation source. UV-vis-NIR diffusion spectra were measured on a Cary 5000 spectrometer in the wavelength range of 250 nm to 2000 nm.
The ZnO powder was mixed with deionized water at a weight ratio of 4:1 to form a paste. The sensors were made by coating the paste on a ceramic tube (4 mm in length,1.2 mm in diameter) to form a thin 10mm zinc oxide film. The tube was installed with a pair of gold electrodes connected with two Pt wires,and a Ni-Cr heating wire inserted into the tube to form an indirect-heated gas sensor. The gas-sensing properties of the sensor were measured by a Chemical Gas Sensor-8 Intelligent Gas Sensing Analysis System (China). The response of the sensor is defined as the ratio of sensor resistance in dry air (Ra) to that in a ta(Rg)et gas ((Rg)) between 250℃ and 500℃,namely,the gas response value is defined asSfrom the calculation of Eq. (1):

Fig. 1 shows the X-ray diffraction (XRD) patterns of the mesoporous ZnO hollow spheres synthesized under differentRw. All products have the Wurtzite structure,and the diffraction peaks can be well indexed to hexagonal ZnO (JCPDS Card No. 3-752). The intense peaks of the XRD pattern indicate that the products are well crystallized. No additional peak was detected by XRD, indicating the superior purity of the ZnO products. The peak intensity of samples from G1 to G3 increases with increasing glycerol content. The broad diffraction peaks in the XRD patterns (Fig. 1) of the ZnO microspheres may imply that the microspheres may be assembled by ZnO nanoparticles.

|
Download:
|
| Fig. 1. XRD patterns of samples G1,G2 and G3,synthesized in a hydrothermal system with anRwratio of 1:10:75,2:10:75 and 3:10:75,respectively. | |
Fig. 2 shows the morphologies of the mesoporous ZnO hollow microspheres. The overview in Fig. 2(a1) shows the welldistributed ZnO microspheres with a uniform diameter of ~2mm,while a3,b3 and c3 of Fig. 2 reveal the hollow structure of ZnO microspheres. Both G2 and G3,obtained under higherRw, show a la(Rg)er dimensional diameter of~9mm,which is greater than that of G1. Interestingly,G1 shows a very rough and mesoporous exterior surface consisting of some serrated protuberances,in which there are numerous mesopores with a diameter of~80 nm and an irregular shape. We can clearly notice that the external surface becomes smoother and solid from sample G1,G2 to G3,with increasingRw. Furthermore,the obvious difference of microsphere size from G1,G2 to G3 can be observed in the fracture section in Fig. 2(a3,b3 and c3). Fig. 2(a3) reveals that the shell of the ZnO hollow sphere is composed of a bundle of nano ZnO rods with a diameter of~80 nm and a length of~800 nm, oriented parallel to the radius direction of the sphere. Fig. 2(a3) shows that one rod with a length of~800 nm extending through the shell of the sphere along the radius direction,but in Fig. 2(c3), many rods with shorter lengths of~200 nm run through the shell end to end. One can conclude that the morphology of the ZnO microsphere can be controlled efficiently by adjusting theRwratio. The formation mechanism can be explained using the Ostwald ripening process [26]. In the primary stage,ZnO microspheres are formed through thermal hydrolysis along with aggregation. During the ripening process,the hollow volume extended symmetrically from the inner core to the sphere surface. The aggregation size increases in the initial stage with increasing amounts of zinc ion precursors. The densification of the microsphere shell in the ripening process causes ZnO nanoparticles to shrink to form mesoporous structures under low ZnO precursor concentration. Therefore,the shape and size of the microspheres can be controlled by adjusting the amount of zinc ion precursors. Due to this unusual structure,the mesoporous ZnO hollow spheres promise a wide application in the fields of gas sensing,photocatalysis,photoluminescence and UV adsorption,etc.

|
Download:
|
| Fig. 2. FESEM photos of samples G1,G2 and G3,synthesized in a hydrothermal system with anRwratio of 1:10:75,2:10:75 and 3:10:75,respectively. | |
The room temperature photoluminescence (PL) spectra of the obtained hollow spheres were recorded under UV excitations at an excitation wavelength of 325 nm. As shown in Fig. 3,the curves display an intense UV emission at~392 nm,which result from the recombination of excitation centers in the ZnO crystal [33]. The blue-green peak at~488 nm is attributed to the defect emission related to oxygen vacancies [34]. The existence of an oxygen vacancy facilitates a high adsorption of oxygen,which increases the response to reductive gases,such as ethanol and acetone. Moreover,with increasing dosage of zinc acetate hydrate used in the hydrothermal system,the intensities of the UV and the blue-green emission remarkably increase and the UV emission peaks shift to short wavelength. These results may be attributed to the high crystallinity and the small size of ZnO spheres obtained in the hydrothermal system with a low concentration of zinc acetate hydrate. The surface morphology is perhaps a possible reason,because the intensity of UV emission increases while the exterior surface becomes smoother from sample G1,G2 to G3.

|
Download:
|
| Fig. 3. Photoluminescence spectra of samples G1,G2 and G3,synthesized in a hydrothermal system with an Rw ratio of 1:10:75,2:10:75 and 3:10:75, respectively. | |
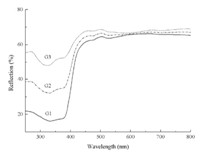
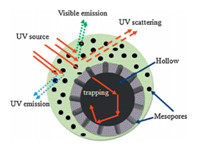
Fig. 4 shows the UV-vis-NIR spectra of the ‘‘as-synthesized’’ hollow ZnO spheres in the range of 250-800 nm,indicating the intensity of the reflected light in the range of 250-400 nm becomes weaker from sample G3,G2 to G1,but shows no difference in the range of 400-800 nm. This implies that intense absorption occurs in UV range to the mesoporous hollow ZnO spheres. The above results disclose that the higher intensity of UV emission at~392 nm accompanies the higher UV scatters in the entire UV band. We note that it seems theoretically contradictory between the results of UV emission and UV scattering reported herein,because in most cases, high emission generally come from high absorbing and low scattering in short UV wavelength. In fact,we can reasonably explain the phenomenon under consideration as a consequence of the special structure of the porous hollow spheres. Fig. 5 shows the schematic diagram of emission,scattering and the trapping mechanism of mesoporous hollow ZnO spheres. The porous hollow sphere can act as a black-body allowing a part of the UV excitation source and emission light to enter into the inner area of the spheres through the mesopores located in its shell and unable to escape. We can conclude that the intensity of photoluminescence emission and UV scattering is regulated by the crystallinity,the crystal size and the porosity of the ZnO spheres in common. Therefore,the more pores, the more UV light trapped by the spheres. One can predict that the porous hollowsphere presents an excellent promise in application of absorption in UV band.
|
Download:
|
| Fig. 4. UV-vis-NIR diffusing spectra of samples G1,G2 and G3,synthesized in a hydrothermal system with an Rw ratio of 1:10:75,2:10:75 and 3:10:75, respectively. | |
|
Download:
|
| Fig. 5. Schematic diagram of emission,diffusion and trapping mechanism of mesoporous hollow ZnO spheres. | |
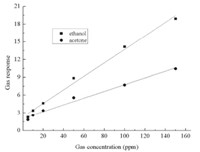
Fig. 6 shows the gas sensing response curves of sensors based on mesoporous hollow ZnO (Sample G1) to ethanol and acetone under different gas concentrations,respectively. With increasing test gas concentration from 5 ppm to 150 ppm,the response values of the gas sensor obviously increase. The response values of the sensor to ethanol at concentrations of 5,10,20,50,100 and 150 ppm are 2.3, 3.4,4.6,8.9,14.1 and 18.9,respectively (Fig. 6a). Fig. 6b shows similar results for sensing acetone,the response values of the sensor are 1.8,2.6,3.4,5.6,7.7 and 10.4 to acetone at concentrations of 5,10,20,50,100 and 150 ppm,respectively.

|
Download:
|
| Fig. 6. Typical response and recovery characteristic curves of sensor based on mesoporous hollow ZnO spheres to ethanol (a) and acetone (b) in the range of 1-150 ppm at 390℃ | |
The insets in Fig. 6 show the response and recovery times of the sensor to 10 ppm ethanol are 2 s and 3 s; and the response and recovery times to 10 ppm acetone are 3 s and 5 s,respectively. The sensing properties of the mesoporous ZnO hollow spheres have been greatly improved compared with that of reported ZnO microspheres without mesopores [35, 36]. Such a fast response and recovery behavior could be attributed to the mesoporous hollow structures,which easily enable gas molecules to adsorb onto the surfaces of the materials. Moreover,the sensor can output a stable response during the testing duration,and we do not observe the saturation phenomenon when the gas concentration is 150 ppm and can conclude that this sensor may be used to detect ethanol and acetone over an extensive concentration range. The linear functional relationship between sensor response and the gas concentration of ethanol and acetone are plotted by a linear fit method in Fig. 7,respectively,demonstrating that the response of the sensor linearly increases with the test gas concentration over the range from 1 ppm to 150 ppm. The linear fit equations are shown below in Eq. (2) to Eq. (3):
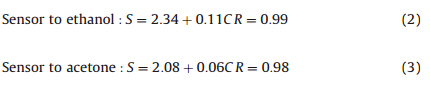
|
Download:
|
| Fig. 7. Linear dependence of the sensor response on the concentrations of ethanol and acetone in the range of 1-150 ppm | |
Mesoporous hollow ZnO microspheres can be successfully synthesized by the one-step facile hydrothermal method,using zinc acetate dihydrate as the starting material and glycerol as the solvent. The morphology of the hollow spheres can be controlled by weight ratio of Zn(CH3COO)2·2H2O:H2O:C3H8O3,and the optimal ratio for synthesis of mesoporous ZnO hollow spheres is 1:10:75. Photoluminescence measurements of the hollow spheres show an intense UV emission at~392 nm and a broad blue-green emission at~488 nm. The mesoporous hollow spheres achieve good improvement of the gas sensing properties to ethanol and acetone gas. The characteristic structure presents an attractive promise in the application of gas sensing,UV emission and absorption.
AcknowledgmentsThe work was jointly supported by the National Natural Science Foundation of China and the Civil Aviation Administration of China (No. 61079010). We are also grateful to the financial supports of the Science and Technology Innovation Guide Funds of Civil Aviation Administration of China (2014),and the Significant Preresearch Funds of Civil Aviation University of China (No. 3122013P001).
| [1] | P.T. Zhao, T. Huang, K.X. Huang, Fabrication of indium sulfide hollow spheres and their conversion to indium oxide hollow spheres consisting of multipore nanoflakes, J. Phys. Chem. C 111 (2007) 12890-12897. |
| [2] | J. Zhang, S.R. Wang, Y. Wang, et al., ZnO hollow spheres: preparation, characterization, and gas sensing properties, Sens. Actuators B: Chem. 139 (2009) 411-417. |
| [3] | K. Watanabe, T. Miyao, K. Higashiyama, H. Yamashita, M. Watanabe, High temperature water-gas shift reaction over hollow Ni-Fe-Al oxide nano-composite catalysts prepared by the solution-spray plasma technique, Catal. Commun. 10 (2009) 1952-1955. |
| [4] | B.Q. Lu, Y.J. Zhu, G.F. Cheng, Y.J. Ruan, Synthesis and application in drug delivery of hollow-core-double-shell magnetic iron oxide/silica/calcium silicate nanocomposites, Mater. Lett. 104 (2013) 53-56. |
| [5] | J. Li, Y. Qin, C. Jin, et al., Highly ordered monolayer/bilayer TiO2 hollow sphere films with widely tunable visible-light reflection and absorption bands, Nanoscale 5 (2013) 5009-5016. |
| [6] | G.H. Wu, A. Milkhailovsky, H.A. Khant, et al., Remotely triggered liposome release by near-infrared light absorption via hollow gold nanoshells, J. Am. Chem. Soc. 130 (2008) 8175-8177. |
| [7] | S.R.A. Raza, Y.T. Lee, Y.G. Chang, et al., Photoelectric probing of the interfacial trap density-of-states in ZnO nanowire field-effect transistors, Phys. Chem. Chem. Phys. 15 (2013) 2660-2664. |
| [8] | C.X. He, B.X. Lei, Y.F. Wang, et al., Sonochemical preparation of hierarchical ZnO hollow spheres for efficient dye-sensitized solar cells, Chem. Eur. J. 16 (2010) 8757-8761. |
| [9] | H. Arami, M. Mazloumi, R. Khalifehzadeh, S.K. Sadrnezhaad, Self-assembled nanostructured ZnO hollow spheres with UVA luminescence, Adv. Appl. Ceram. 108 (2009) 73-77. |
| [10] | Y.L. Chen, C.E. Zhang, C. Deng, et al., Preparation of ZnO/GO composite material with highly photocatalytic performance via an improved two-step method, Chin. Chem. Lett. 24 (2013) 518-520. |
| [11] | D. Barreca, D. Bekermann, E. Comini, et al., 1D ZnO nano-assemblies by plasma-CVD as chemical sensors for flammable and toxic gases, Sens. Actuators B: Chem. 149 (2010) 1-7. |
| [12] | G.Q. Yang, X.P. Zou, X.M. Meng, et al., Large-scale synthesize ZnO micro/nano rods fabricated from aqueous solutions at low temperature, Adv. Mater. Res. 123-125 (2010) 715-718. |
| [13] | D.L. Guo, L.H. Tan, Z.P. Wei, H.Y. Chen, T. Wu, Density-controlled synthesis of uniform ZnO nanowires: wide-range tunability and growth regime transition, Small 9 (2013) 2069-2075. |
| [14] | W.J. Liu, X.Q. Meng, Y. Zheng, W. Xia, Synthesis and photoluminescence properties of ZnO nanorods and nanotubes, Appl. Surf. Sci. 257 (2010) 677-679. |
| [15] | J.B. Lian, Z.M. Ding, F.L. Kwong, D.H.L. Ng, Template-free hydrothermal synthesis of hexagonal ZnO micro-cups and micro-rings assembled by nanoparticles, CrystEngComm 13 (2011) 4820-4822. |
| [16] | S.H. Wei, Y. Zhang, M.H. Zhou, Toluene sensing properties of SnO2-ZnO hollow nanofibers fabricated from single capillary electrospinning (vol. 151, p. 895, 2011), Solid State Commun. 152 (2012) 329. |
| [17] | Z.Y. Zhang, X.H. Li, C.H. Wang, et al., ZnO hollow nanofibers: fabrication from facile single capillary electrospinning and applications in gas sensors, J. Phys. Chem. C 113 (2009) 19397-19403. |
| [18] | Z.R. Shen, J.G. Wang, P.C. Sun, D.T. Ding, T.H. Chen, Fabrication of lanthanide oxide microspheres and hollow spheres by thermolysis of pre-molding lanthanide coordination compounds, Chem. Commun. 13 (2009) 1742-1744. |
| [19] | H.M. Zhang, C. Xu, P.K. Sheng, et al., Synthesis of ZnO hollow spheres through a bacterial template method and their gas sensing properties, Sens. Actuators B: Chem. 181 (2013) 99-103. |
| [20] | Y. Tian, J.C. Li, H. Xiong, J.N. Dai, Controlled synthesis of ZnO hollow microspheres via precursor-template method and its gas sensing property, Appl. Surf. Sci. 258 (2012) 8431-8438. |
| [21] | J.H. Wang, N. Shi, Y. Qi, M.L. Liu, Reverse micelles template assisted fabrication of ZnO hollow nanospheres and hexagonal microtubes by a novel fast microemulsion-based hydrothermal method, J. Sol-Gel. Sci. Technol. 53 (2010) 101-106. |
| [22] | M. Wang, X.L. Cao, L.J. Wang, L.D. Zhang, Template-free fabrication of porous zinc oxide hollow spheres and their enhanced photocatalytic performance, J. Porous Mater. 17 (2010) 79-84. |
| [23] | C.H. Deng, H.M. Hu, G.Q. Shao, C.L. Han, Facile template-free sonochemical fabrication of hollow ZnO spherical structures, Mater. Lett. 64 (2010) 852-855. |
| [24] | X.S. Chen, X.Y. Jing, J. Wang, et al., Self-assembly of ZnO nanoparticles into hollow microspheres via a facile solvothermal route and their application as gas sensor, CrystEngComm 15 (2013) 7243-7249. |
| [25] | X.M. Ma, X.T. Zhang, L. Yang, et al., An unusual temperature gradient crystallization process: facile synthesis of hierarchical ZnO porous hollow spheres with controllable shell numbers, CrystEngComm 16 (2014) 7933-7941. |
| [26] | R.L. Penn, J.F. Banfield, Imperfect oriented attachment: dislocation generation in defect-free nanocrystals, Science 281 (1998) 969-971. |
| [27] | Y. Chang, J.J. Teo, H.C. Zeng, Formation of colloidal CuO nanocrystallites and their spherical aggregation and reductive transformation to hollow Cu2O nanospheres, Langmuir 21 (2005) 1074-1079. |
| [28] | B. Liu, H.C. Zeng, Symmetric and asymmetric Ostwald ripening in the fabrication of homogeneous core-shell semiconductors, Small 1 (2005) 566-571. |
| [29] | B.P. Jia, L. Gao, Morphological transformation of Fe3O4 spherical aggregates from solid to hollow and their self-assembly under an external magnetic field, J. Phys. Chem. C 112 (2008) 666-671. |
| [30] | Y.D. Yin, R.M. Rioux, C.K. Erdonmez, et al., Formation of hollow nanocrystals through the nanoscale Kirkendall effect, Science 304 (2004) 711-714. |
| [31] | X. Liang, X. Wang, Y. Zhuang, et al., Formation of CeO2-ZrO2 solid solution nanocages with controllable structures via Kirkendall effect, J. Am. Chem. Soc. 130 (2008) 2736-2737. |
| [32] | X. Wang, Y.J. Yang, Y. Ma, J.N. Yao, Controlled synthesis of multi-shelled transition metal oxide hollow structures through one-pot solution route, Chin. Chem. Lett. 24 (2013) 1-6. |
| [33] | J. Rao, A. Yu, C.L. Shao, X.F. Zhou, Construction of hollow and mesoporous ZnO microsphere: a facile synthesis and sensing property, ACS Appl. Mater. Interfaces 4 (2012) 5346-5352. |
| [34] | M. Chen, Z.H. Wang, D.M. Han, F.B. Gu, G.S. Guo, Porous ZnO polygonal nanoflakes: synthesis, use in high-sensitivity NO2 gas sensor, and proposed mechanism of gas sensing, J. Phys. Chem. C 115 (2011) 12763-12773. |
| [35] | X.W. Li, P. Sun, T.L. Yang, et al., Template-free microwave-assisted synthesis of ZnO hollow microspheres and their application in gas sensing, CrystEngComm 15 (2013) 2949-2955. |
| [36] | P. Song, Q. Wang, Z.X. Yang, Acetone sensing characteristics of ZnO hollow spheres prepared by one-pot hydrothermal reaction, Mater. Lett. 86 (2012) 168-170." |




