b School of Pharmaceutical Engineering & Life Science, Changzhou University, Changzhou 213164, China
Spirocyclicoxindoles are important structural motifs found in numerous natural products and synthetic pharmaceuticals with biological and pharmaceutical activities. Thus,many efficient methodologies have been developed for the rapid construction of structurally diverse spirooxindoles [1]. Among those valuable methods,1,3-dipolar cycloaddition remains as one of the most powerful tools by using relatively readily available precursors to achieve this goal [2]. Meanwhile,azomethine imines have been employed as efficient 1,3-dipoles in various cycloadditions with dipolarophiles [3, 4]. In particular,azomethine imines can also serve as potential 1,3-dipoles in constructing spirocyclicoxindoles [5]. Recently,Wang and co-workers reported two elegant approaches in the use ofN,N' -cyclic azomethine imines in this reaction in a stereoselective as well as an enantioselective way [5a, 5b]. Despite these advances,the development of efficient approaches to synthesize spirocyclicoxindoles from N,N' -cyclic azomethine imines in the presence of a cheap catalyst under simple reaction conditions is still highly desirable. Herein,we wish to report the synthesis of spirocyclicoxindoles in moderate to high yields and good stereoselectivities from methyleneindolinones andN,N' -cyclic azomethine imines catalyzed by Cu(OAc)2under mild conditions. 2. Experimental
N,N' -cyclic azomethine imines 7were prepared according to literature [4a, 6]. 1HNMR and 13C NMR were recorded on Bruker 400 MHz,500 MHz spectrometers in CDCl3or DMSO-d6solution. HRMS were performed using electrospray ionization (ESI) with Bruker Daltonics,Inc. or APEXIII 7.0 TESLA FTMS. Melting points were determined on a SGW X-4B melting point apparatus. HPLC analysis was performed on an Agilent 1260 (UV detection monitored at 254 nm). Theeevalue for 6aa was obtained using a Chiralpak AD-H column. The spectral data and spectra of all compounds can be found in Supporting Information.
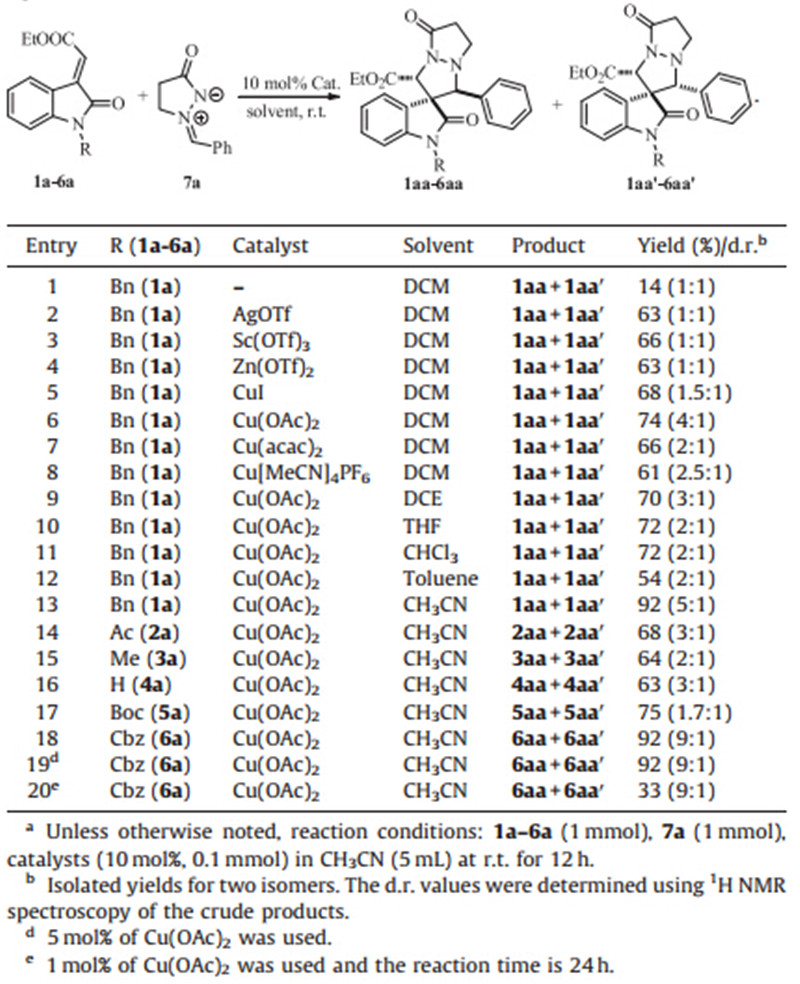
Initially,we utilized N-benzyl methyleneindolinones 1a and azomethine imine 7a as model substrates in dichloromethane (DCM) in the presence of various Lewis acids at room temperature. We found that Cu(OAc)2gave the best result and the corresponding product was obtained in 74% isolated yield with 4:1 stereoselectivity in 10 h (Table 1,entry 6). The use of AgOTf,Sc(OTf)3, Zn(OTf)2,CuI,Cu(acac)2and Cu[CH3CN]4PF6gave poor selectivity (Table 1,entries 2-5 and 7-8). Solvent screening showed that acetonitrile (CH3CN) was the most suitable solvent according to the reactivity and selectivity (5:1) (Table 1,entry 13). Based on these results,we next focused on investigating the influence of different substituents ofN-substituted methyleneindolinones in this reaction. To our delight,theN-Cbz (benzylcarboxy carbamate) substituted methyleneindolinone 6a gave the corresponding product in excellent yield and good stereoselectivity (9:1) (Table 1,entry 18). Finally,when the catalyst loading was reduced to 5 mol%,the same result was obtained (Table 1,entry 19). However,reduced the catalyst loading to 1 mol% gave low yield even under longer reaction time (Table 1,entry 20).
| Table 1 Optimization of reaction conditions.a |
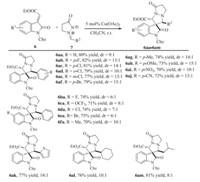
With the optimal reaction conditions in hand,the scope of substrates was then investigated (Scheme 1). Generally,the reaction proceeded smoothly to afford the desired products in moderate to high yields,with good stereoselectivities for most cases. First,the reaction of methyleneindolinone 6a with a wide range ofN,N'-cyclic azomethine imines 7a-7mwere examined. The results indicated that the positions and electronic properties of substituents on the aromatic ring had a limited effect on the efficiency of this transformation. The spirocyclicoxindoles were obtained in moderate to high yields with good stereoselectivities (70-82% yield,9:1 to 15:1 d.r.) (Scheme 1,6aa to 6aj). Notably,the hetero aryl-substituted azomethine imine 7k was also tolerated in the reaction and delivered the corresponding product in 77% yield and 14:1 d.r. value (Scheme 1,6ak). In addition,alkyl-substituted N,N' -cyclic azomethine imines7l and7 mwere also examined. The corresponding products were obtained in 76% and 81% yield,10:1 and 8:1 dr. value respectively. Next,the influence of substituted groups on the methyleneindolinones 6 was investigated. However, no significant difference was observed for the reactivity and stereoselectivity (Scheme 1,6ba to 6fa).
|
Download:
|
| Scheme 1.Substrate scope. Reaction conditions:6(1 mmol),7(1 mmol),Cu(OAc)2 (5 mol%,0.05 mmol) in CH3CN (5 mL) at room temperature for 12 h under N2 atmosphere. Yields refer to isolated yields; the d.r. ratios were determined by NMR analysis of crude products. | |
Further investigation on this reaction in an enantioselective way was carried out (Scheme 2). The use of commercially available oxazoline ligands L1andL2 in combination of Cu(OAc)2gave the product in 11%eeand 18%eerespectively.iPr-Phosferrox afforded 6aa in 80% yield and 48%ee. Unfortunately,we did not obtain high enantioselectivities after screening a series of chiral ligands.

|
Download:
|
| Scheme 2.Asymmetric copper-catalyzed [3 + 2] cycloadditions. | |
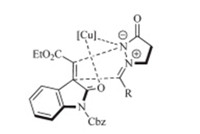
Based on the above results,we proposed here a possible transition state to explain the stereochemistry of the cycloaddition (Scheme 3). The electron-deficient methyleneindolinone is activated by Cu(OAc)2 and stabilized forward. Concurrently,the azomethine imine is also activated by Cu(OAc)2and this 1,3-dipole may attack from the backside to proceed in a formal [3 + 2] cycloaddition to give the target product.
|
Download:
|
| Scheme 3.Proposed transition state. | |
In conclusion,we have described a highly selective coppercatalyzed [3 + 2] cycloaddition of methyleneindolinones and N,N' -cyclic azomethine imines to afford spiro[pyrazolidin-3,3' -oxindoles] in moderate to high yields with good stereoselectivities. This protocol featured simple and mild reaction conditions and catalyzed by cheaper Cu(OAc)2.
AcknowledgmentsWe gratefully acknowledge the National Natural Science Foundation of China (No. 21172023),A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and STD of Jiangsu Province for their financial supports.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.11.015.
| [1] | (a) R.M. Williams, R.J. Cox, Paraherquamides, brevianamides, and asperparalines: laboratory synthesis and biosynthesis. An interim report, Acc. Chem. Res. 36 (2003) 127-139; (b) F. Zhou, Y.L. Liu, J. Zhou, Catalytic asymmetric synthesis of oxindoles bearing a tetrasubstituted stereocenter at the C-3 position, Adv. Synth. Catal. 352 (2010) 1381-1407; (c) G.S. Singh, Z.Y. Desta, Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks, Chem. Rev. 112 (2012) 6104-6155; (d) L. Hong, R. Wang, Recent advances in asymmetric organocatalytic construction of 3,3'-spirocyclic oxindoles, Adv. Synth. Catal. 355 (2013) 1023-1052; (e) L. Chen, X.P. Yin, C.H. Wang, J. Zhou, Catalytic functionalization of tertiary alcohols to fully substituted carbon centres, Org. Biomol. Chem. 12 (2014) 6033-6048; (f) H.F. Zhang, Z.Q. Ye, G. Zhao, Enantioselective synthesis of functionalized fluorinated dihydropyrano [2,3-c]pyrazoles catalyzed by a simple bifunctional diaminocyclohexane-thiourea, Chin. Chem. Lett. 25 (2014) 535-540. |
| [2] | L.M. Stanley, M.P. Sibi, Enantioselective copper-catalyzed 1,3-dipolar cycloadditions, Chem. Rev. 108 (2008) 2887-2902. |
| [3] | W. Chen, W. Du, Y.Z. Duan, et al., Enantioselective 1,3-dipolar cycloaddition of cyclic enones catalyzed by multifunctional primary amines: beneficial effects of hydrogen bonding, Angew. Chem. Int. Ed. 46 (2007) 7667-7670. |
| [4] | (a) A. Chan, K.A. Scheidt, Highly stereoselective formal [3 + 3] cycloaddition of enals and azomethine imines catalyzed by N-heterocyclic carbenes, J. Am. Chem. Soc. 129 (2007) 5334-5335; (b) R. Na, C. Jing, Q. Xu, et al., Phosphine-catalyzed annulations of azomethine imines: Allene-dependent [3 + 2], [3 + 3], [4 + 3], and [3 + 2 + 3] pathways, J. Am. Chem. Soc. 133 (2011) 13337-13348; (c) Z.L. He, H.L. Teng, C.J. Wang, Fulvenes as effective dipolarophiles in copper(I)-catalyzed [6 + 3] cycloaddition of azomethine ylides: asymmetric construction of piperidine derivative, Angew. Chem. Int. Ed. 52 (2013) 2934-2938; (d) M.C. Tong, X. Chen, H.Y. Tao, C.J. Wang, Catalytic asymmetric 1,3-dipolar cycloaddition of two different ylides: facile access to chiral 1,2,4-triazinane frameworks, Angew. Chem. Int. Ed. 52 (2013) 12377-12380; (e) Y. Qian, P.J. Zavalij, W. Hu, M.P. Doyle, Bicyclic pyrazolidinone derivatives from diastereoselective catalytic [3 + 3]-cycloaddition reactions of enoldiazoacetates with azomethine imine, Org. Lett. 15 (2013) 1564-1567; (f) X. Fang, J. Li, H.Y. Tao, C.J. Wang, Highly diastereoselective DABCO-catalyzed[3 + 3]-cycloaddition of 1,4-dithiane-2,5-diol with azomethine imines, Org. Lett. 15 (2013) 5554-5557. |
| [5] | (a) G. Zhu, W. Sun, C. Wu, et al., Base-catalyzed diastereoselective [3 + 3] annulation of 3-isothiocyanatooxindoles and azomethine imine, Org. Lett. 15 (2013) 4988-4991; (b) L. Hong, M. Kai, C. Wu, et al., Enantioselective 1,3-dipolar cycloaddition of methyleneindolinones and N,N'-cyclic azomethine imines, Chem. Commun. 49 (2013) 6713-6715; (c) X. Jiang, Y. Sun, J. Yao, et al., Core scaffold-inspired concise synthesis of chiral spirooxindole-pyranopyrimidines with broad-spectrum anticancer potency, Adv. Synth. Catal. 354 (2012) 917-925; (d) W. Sun, G. Zhu, C. Wu, et al., Organocatalytic diastereo-and enantioselective 1,3-dipolar cycloaddition of azlactones and methyleneindolinones, Angew. Chem. Int. Ed. 52 (2013) 8633-8637. |
| [6] | R. Shintani, Y.T. Soh, T. Hayashi, Rhodium-catalyzed asymmetric arylation of azomethine imines, Org. Lett. 12 (2010) 4106-4109." |