With the development of microelectromechanical systems (MEMS) technology,more and more materials have been applied to MEMS in order to achieve effective microsystems [1]. Encapsulation is one of the key technologies for the industrialization of MEMS technologies since the reliability of MEMS components greatly depends on the encapsulation quality [2]. At present,the bonding technology is one of the most challenging technologies among MEMS technologies,and the relatively mature bonding technologies include anodic bonding technology,silicon-silicon bonding technology,gold-silicon co-melting bonding technology,aluminumsilicon co-melting bonding technology,and cold welding bonding technology,of which anodic bonding technology has been widely applied [3]. Anodic bonding can achieve solid connection between glass and silicon or metals,as well as the alloy thereof,under the action of a static electric field,and such technology also offers a simple process,high bonding strength,good sealing performance, etc. [4]. However,anodic bonding requires the encapsulating materials to have ionic conduction in order to ensure the mutual diffusion between positive and negative ions under the action of the electric field during the bonding process. As the cathodic bonding material,only Pyrex7740 glass produced by US Corning Corporation can meet the bonding requirements of the present anodic bonding process. As a kind of solid electrolyte material,Pyrex7740 glass contains alkali metal ions,thus basically satisfying the ionic conduction requirement [5]. However,Pyrex7740 glass can achieve ideal bonding only under high bonding temperature that accordingly causes great bonding stress and damages the front-end process and the structure of MEMS components; meanwhile,Pyrex7740 glass is high in cost. Solid polymer electrolyte (also called ionic conduction polymer) is a kind of new solid electrolyte material developed in recent years [6]. Because solid polymer electrolyte materials feature ionic conductivity,viscoelasticity,small thermal expansion coefficient,workability,etc.,both the bonding temperature and the residual encapsulation stress can be reduced during the bonding process,thus improving the encapsulation quality of MEMS components [7]. At present,there is no research on the encapsulation achieved through the bonding of solid electrolyte polymer materials and metals,thus limiting the application of polymer functional materials in MEMS components [8]. Therefore,the research results of this paper are significant for expanding the application of anodic bonding technology,developing new uses for polymer materials,and promoting the development of MEMS. 2. Experimental
Preparation of PEO-LiClO4 used for anodic bonding: Raw materials of this experiment: solid polyethylene oxide (PEO) powder with relative molecular mass of 5,000,000,purity>99.36%, granularity<80mm,which was pre-dried in vacuum at 50℃; LiClO4,analytical reagent,purity >99.6%,granularity<50mm, which was pre-dried in vacuum at 150℃. The above two powders were put into a ball milling tank in different proportions with absolute ethyl alcohol as the grinding agent and agate balls 8 mm, 5 mm,and 3 mm in diameter as the ball milling media. The proportion of the three kinds of balls was 1:1:1,the proportion of the balls and the materials was 8:1,the rotating speed was 280 rpm,the ball milling time was 12 h,and the ball milling operations were completed on a QM-3SP planetary high-energy ball mill. After the ball milling process,the powdered material was dried and sieved to obtain a fine powder,which was then pressed into a round sheet with a diameter of 25 mm and the thickness of 3 mm in a cylinder type mold cavity.
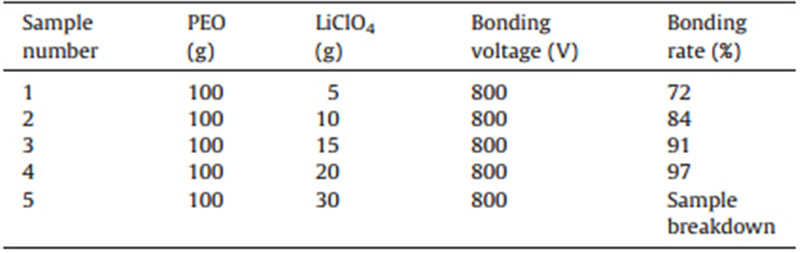
Anodic bonding experimental device: The device for the anodic bonding experiment of PEO-LiClO4 and metallic aluminum is shown in Fig. 1,and the bonding device is mainly composed of a high voltage power supply,bonding room,and a heating and temperature-regulating device,wherein the bonding room is internally equipped with a heating plate,lower and upper electrodes,an assembling platform,bonding power supply,and a temperature-regulating cabinet; a 10-1000 V continuously regulated DC pulse bandwidth filtering power supply is adopted as the bonding power supply; TRC current protection,microcomputer data collection,as well as an arithmetical unit are also configured; a SiC heating element and thermocouple temperature monitor are adopted for the heating furnace; and a triaxial parallel locating device and three-dimensional adjustment machinery are adopted for the assembly platform.
|
Download:
|
| Fig. 1. Schematic diagram of anodic bonding device. | |
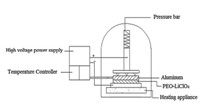
According to XRD analysis,increasing the content of the lithium salt has great impact on the conductivity of the polymer electrolyte; here Fig. 2(a) is the XRD Graph of PEO,Fig. 2(b) is the XRD Graph of PEO-LiClO4generated by the ball-milled PEO and LiClO4with the mass content of 5% through complexation reaction, and Fig. 2(c) is the XRD Graph of PEO-LiClO4generated by the ballmilled PEO and LiClO4 with the mass content of 10% through complexation reaction. The scanning scope (2θ) is 10-508. Two obvious diffraction peaks respectively appear at 2θ=19℃ and 2θ=23℃ in the graphs. According to the analysis,the addition of lithium salt does not change the position of the diffraction peak, but compared to PEO,the strength of the diffraction peak thereof is reduced and the strength of the diffraction peak is more greatly reduced along with the increment of LiClO4. Since the conductivity of the polymer electrolyte mainly relies on the proportion of the amorphous phase high-elastic area,such phenomenon indicates that the increment of LiClO4 can effectively obstruct the crystallization of the solid polymer electrolyte,thus increasing the content of the amorphous area and favorably improving the room-temperature conductivity thereof.
|
Download:
|
| Fig. 2. XRD spectra of PEO-LiClO4 | |
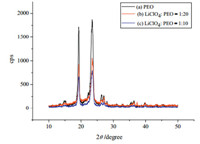
According to DSC results shown in Fig. 3(a),the melting temperature of the pure PEO is 80℃,and after adding LiClO4and PEO for complexation,the thermal properties of PEO significantly change. Fig. 3(b) is the DSC curve of PEO-LiClO4generated by the ball-milled PEO and LiClO4with the mass content of 5% through complexation reaction,and Fig. 3(c) is the DSC curve of PEO-LiClO4 generated by the ball-milled PEO and LiClO4with the mass content of 10% through complexation reaction. An obvious peak is located at the position of 74℃ in Fig. 3(b) while an obvious peak is located at the position around 65℃ in Fig. 3(c),which is regarded as the transition point of PEO from the crystalline state to the amorphous state. According to the analysis,the addition of LiClO4reduces the glassy state transformation temperature of the solid polymer electrolyte and such transformation temperature is more greatly reduced along with increasing LiClO4,this is because after the complexation of LiClO4and PEO,part a portion of the hydrogen bonds are shielded,and the regular arrangement of PEO molecular chain is destroyed,thus reducing the crystallization property of the molecules and the size of the crystals so that imperfect crystals are formed and the glassy state transformation temperature thereof is reduced. Therefore,the analysis indicates that LiClO4 has an obvious inhibitory effect on PEO crystallization,which is further favorable for the diffusion of Li-ion during the anodic bonding process under low temperature.
|
Download:
|
| Fig. 3. DSC of PEO-LiClO4 | |
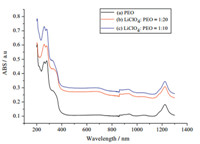
Fig. 4(a) is the ultraviolet absorption spectrum test of PEO. At 300 nm,PEO has significant ultraviolet absorption. Fig. 4(b) and (c) shows the ultraviolet absorption spectrum test of PEO-LiClO4with different lithium salt contents. According to the above figures,the original peak of the absorbency around 300 nm was significantly strengthened along with the gradual increase of LiClO4content,but the position of the peak is not changed,suggesting a gradually reduced light transmittance. The change of the absorption peak illustrates that PEO and LiClO4will complex to some extent when LiClO4 is added to the PEO,the complexing degree depends on the ratio of PEO/Li+ . The coordination function of Li+ and ether oxygen atoms in the chain of the PEO molecules will enhance with the increasing content of Li+ in the PEO molecules,which make the C=O of the PEO molecules form a large complex group around Li+ , thus increasing the absorbency,significantly reducing the light transmittance and continuously improving the complexation degree.
|
Download:
|
| Fig. 4. UV absorption spectra of PEO-LiClO4 | |
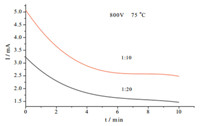
PEO-LiClO4 and metallic aluminum foil are overlapped and then assembled between the cathode and the anode wherein PEO- LiClO4 is connected to the cathode and the aluminum foil is connected to the anode. The preset voltage is 800 V,the preheating temperature is 75℃,and the bonding time is 10 min. According to Fig. 5,weak current is generated in the bonding circuit under the action of the strong static electric field,and at the beginning of the bonding,the current instantaneously rises to the maximum value, and then the ion migration trends to be saturated and the current gradually declines with time; When the bonding parameter is determined,the ion migration increases along with LiClO4content, thus increasing current.
|
Download:
|
| Fig. 5. Currentversustime curve. | |
The binding rate is necessarily regarded as an important index to evaluate the connection quality of the interface so as to further evaluate the bonding quality. The ultrasonic inspection and measurement can be used to calculate the binding rate. Among the bonding samples,the positive and negative repeated areas of the transition layers on the bonding samples can be used as the basis for calculating the effective binding rate. According to Table 1,under certain bonding conditions,the bonding rate increases with lithium salt content; this is because as the complexratio increases,the mutually diffused ions and the binding area also increase. But,beyond a threshold lithium content,the excessive current will break down the test pieces.
| Table 1 Impact of different complex-ratios on bonding rate. |
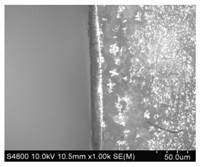
The scanning electron microscope results for the anodic bonding interface of PEO-LiClO4 and aluminum are shown in Fig. 6. According to this figure,the bonding interface is in good condition,without any stripping phenomenon and obvious interspace phenomenon,and an obvious transition area with the width of 5mm exists at the interface of PEO-LiClO4and aluminum. According to the analysis,a great static electric field attraction is generated at the interface of aluminum and PEO,which enables Liion in the solid polymer electrolyte to move toward the cathode under the action of the strong static electric field and enables aluminum to diffuse in PEO,thus causing the close contact of the two bonding interfaces to form the permanent connection of solid polymer electrolyte-diffusion transition layer-aluminum.
|
Download:
|
| Fig. 6. SEM of anodic bonding interface. | |
In this study the ionic conduction polymer solid electrolyte PEO-LiClO4prepared through the high-energy ball milling method is applied to the anodic bonding encapsulation technology. The test analyses of XRD,DSC and ultraviolet absorption spectrum sufficiently indicate that the addition of LiClO4allows C=5 bonds in large PEO molecules to be combined with Li-ion through complexation reaction,and the complexation degree is continuously improved along with the increase of Li+ ,thus effectively obstructing the crystallization of the solid polymer electrolyte, increasing the amorphous areas,reducing the glassy state transformation temperature of the solid polymer electrolyte, and favorably improving the room-temperature conductivity. The results of the anodic bonding experiment show that under certain bonding condition,the larger the complex-ratio is,the larger the bonding current is and the higher the bonding rate is. The generation of the interface transition layer is the main reason for achieving the bonding of PEO-LiClO4and metallic aluminum.
AcknowledgmentsThis study was supported by the National Natural Science Foundation of China (No. 51275332) and the Natural Science Foundation for Young Scientists of Shanxi Province,China (No. 2014021025-2).
| [1] | R. Saha, N. Fritz, S.A. Bidstrup-Allen, P.A. Kohl, Packaging-compatible wafer level capping of MEMS devices, Microelectron. Eng. 104 (2013) 75-84. |
| [2] | F.L. Zhu, Research on Some Basic Issues of MEMS Packaging Based on Process Mechanics, Huazhong University of Science & Technology, 2007. |
| [3] | D.M. Zhang, Z.C. Ye, G.P. Ding, Developing trend of bonding technology for MEMS, Electron. Process Technol. 6 (2005) 315-318. |
| [4] | A.C. Lapadatu, H. Jakobsen, Two-anodic bonding, in: V. Lindroos, M. Tilli (Eds.), Handbook of Silicon Based MEMS Materials and Technologies, 2010, pp. 513-520 (Chapter 30). |
| [5] | N. Gao, Z.G. Chen, S.T. Wang, X.L. Sui, D.D. Gu, Research on PEI-PEO based solid state polymer electrolyte, J. Harbin Inst. Technol. 43 (2011) 654-659. |
| [6] | S.T. Ren, H.F. Chang, T. Zheng, et al., HBPS-PEO multi-arm star polymer electrolytes and their ionic conductivity, Acta Polymer Sin. (2013) 1064-1071. |
| [7] | D.H. Xiong, J.S. Cheng, H. Li, W. Deng, K. Ye, Anodic bonding of glass-ceramics to stainless steel coated with intermediate SiO2 layer, Microelectron. Eng. 87 (2010) 1741-1746. |
| [8] | N. Voigt, L. van, Wüllen, The effect of plastic-crystalline succinonitrile on the electrolyte system PEO:LiBF4: insights from solid state NMR, Solid State Ionics 260 (2014) 65-75." |