b School of Chemistry and Chemical Engineering, Qufu Normal University, Qufu 273165, China;
c Key Lab of Applied Chemistry of Zhejiang Province, Department of Chemistry, Zhejiang University, Hangzhou 310028, China
The structure of indoline can be found extensively in natural alkaloids with biological activity and pharmaceutical molecules [1, 2, 3]. Indolines can be obtained by direct reduction of indoles or ring closure through intermolecular amination reaction [4, 5]. Generally speaking,the direct hydrogenation of indoles is the most straightforward and powerful method to obtain indulines in terms of simplicity and atom efficiency. Despite the progress achieved in the direct hydrogenation of indoles and other N-containing compounds in past decades,efficient hydrogenation of simple unprotected indole remains a longstanding challenge because of the following troubles [6, 7, 8]: (1) low reactivity of the highly resonance-stabilized indole ring,necessitating harsh conditions such as high temperature and high hydrogen pressure; (2) difficulties in achieving high chemo-selectivity because of overhydrogenation and polymerization reactions and (3) deactivation and/or poisoning of catalyst arising from indoline molecule.
Over the past decades,continuous efforts have been devoted to the reduction of indoles to indolines using hydrides [4, 9]. When the typical reducing agent of NaBH3CN is utilized [10, 11, 12], indolines can be obtained with satisfactory yield,but such methodology requires superstoichiometric NaBH3CN and generates a large amount of waste. In fact,other methods using hydride will encounter the same problems in application,including triethylamine/borane [13],Et3SiH/trifluroacetic acid [14], PH2SiH2/B(C6F5)3 [15],H3SiPh/[Rh(nbd)(PPh3)2]PF6 [16],formic acid [17],polymethylhydrosiloxane [18],and NaBH4/acetic acid [19].
Catalytic hydrogenation using hydrogen gas as the reducer is a more attractive protocol with almost no generation of waste. But as mentioned above,how to obtain high chemoselectivity of indoline is still a challenge [20]. Although homogeneous catalysts involving the use of Ru [21, 22, 23],Rh [24, 25],Ir [26],and Pd complexes [27, 28] are often reported in the literature,they are commonly limited to asymmetric hydrogentation of N-protected or substituted indoles. Compared with homogeneous catalysts,heterogeneous catalysts are relatively stable in air and water and easy to store and recycle [29, 30, 31]. Currently,there are only a few reports on heterogeneous hydrogenation of indole to indoline. Shaw [32] first reported the catalytic heterogeneous hydrogenation of unprotected indole using hydrogen gas. But harsh conditions such as hydrogen pressure of 150 bar and temperature of 227℃ were required to give a moderate yield. Recently,To ¨ro ¨k [33] reported the hydrogenation of unprotected indoles with relatively expensive Pt/ C in acidic solutions at room temperature and hydrogen pressure of 30 bar giving 100% yield of indoline. However,when Pt/C was replaced with relatively inexpensive Pd/C,the conversion dropped to only 56% even at hydrogen pressure of 50 bar. In addition,they reported a new reducing system of Ni-Al alloy and water with the best yield of 83% [34]. Fang [35] applied Ru nanoparticles immobilized on poly (4-vinylpyridine) to hydrogenate indole at 150℃ and H2 pressure of 50 bar giving a yield less than 25%. Clarisse [36] used PdO2for hydrogenation of indole in hexafluoroisopropanol,with the yield of 72% at H2pressure of 7 bar and 50℃. Beckers [37] screened 72 multimetallic nanoparticle catalysts supported on metal oxides for hydrogenation of indole, and all the experimental catalysts demonstrated low catalytic activity with conversions less than 18%. Other substantial works on heterogeneous hydrogenation of N-protected and ring-substituted indole derivatives are also reported in the literatures [18, 36, 38].
In our previous works [39, 40, 41, 42, 43, 44],serials of nitrogen doped carbon materials were prepared as support for palladium to make heterogeneous catalysts of Pd@CxNy. They were extensively utilized in the selective hydrogenation of unsaturated aromatic compounds of phenol and quinoline [42, 43, 44, 45]. Continuing our efforts toward developing heterogeneous hydrogenation of heterocycles,herein we report a robust methodology for highly chemoselective hydrogenation of unprotected indole to indoline. The reaction is catalyzed by Pd/CN0.132 and most importantly occurs near room temperature of 40℃ and atmospheric hydrogen gas with water as solvent. 2. Experimental
2.1. Catalyst preparation
All the chemicals were purchased from Sigma-Aldrich,Merck, or Aladdin chemical reagents Co.,Ltd. (Shanghai,China). The chemicals were used as received without further purification. The catalyst Pd@CN0.132 was synthesized according to our previous procedure [43],with the systematical characterization results of Element analysis,Raman spectrum,XRD,XPS,and HRTEM also illustrated. Typically,CN0.132was dispersed in deionized water by ultrasonication,followed by addition of aqueous HCl-solubilizing PdCl2 solution. After treatment in ultrasound for 10 min,NaBH4 aqueous solution was added and further kept in ultrasound for another 30 min. Finally,Pd@CN0.132was separated by filtration, washed with distilled water five times,and then dried at 80℃ overnight in a vacuum oven. The supported Pd nanoparticles were well-dispersed and existed mainly as Pd0 (~71%) on the surface of the catalyst [39]. The high nitrogen content in the texture carbon is suitable for stabilizing highly dispersed Pd0 nanoparticles and prevents their reoxidation. Such characteristics were helpful for the selective hydrogenation of indole. 2.2. Hydrogenation of indole
The reaction was conducted in a Schlenk flask (25 mL). A typical procedure was as follows: 60 mg indole,25 mg Pd@CN0.132, 0.614 mmol H3PO4,and 5 mL water were placed in a flask. The flask was purged with H2to remove the air 3 times and then stirred magnetically at 1500 rpm under H2 atmosphere (connected to a hydrogen balloon) at 40℃. After reaction,the conversion and selectivity of the reaction were determined by chromatographic analysis using a SHIMADZU GC-2011 with FID detector. 3. Results and discussion
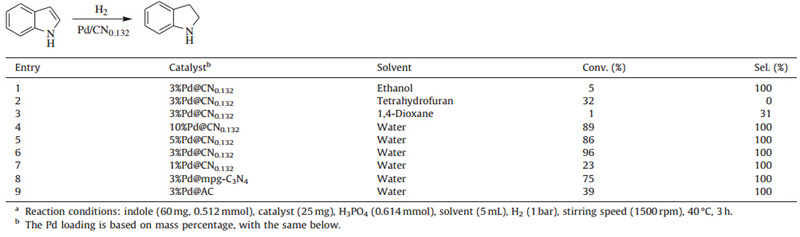
Initially,reaction solvents were investigated by using 3% Pd@CN0.132as catalyst with the results listed in Table 1 (entries 1-3,6). Among the used solvents,water demonstrated the best results in both conversion and selectivity. It is well known that water is a desirable solvent for chemical reactions considering its cost,safety,and negligible environmental impact. We therefore conducted the reactions in water as solvent. Subsequently,with various loading contents of palladium,the effects of heterogeneous catalysts containing 1%,3%,5%,and 10% Pd on the hydrogenation performance were compared (Table 1,entries 4-7). It is evident that the catalyst with 3% Pd content demonstrates the best performance,with the conversion of 96% and selectivity of 100% (entry 6). If the Pd content is reduced to 1%,the conversion sharply decreases to only 23% (entry 7). On the contrary,if the Pd content is further increased to 5%,or even as large as 10%,the conversion does not increase accordingly,but 86% and 89% yields were obtained respectively,which were lower than that obtained with 3% Pd content catalyst (entries 4 and 5). These results disclose that Pd content influences the catalytic activity and 3% Pd content is efficient for the present hydrogenation process. The lower Pd content leads to fewer active sites,but the higher Pd content may lead to the formation of large palladium clusters. Since only the surface palladium atoms can become active sites,the cluster of palladium atoms cannot supply more active sites,so the catalytic activity does not increase any more.
| Table 1 Chemoselective hydrogenation of indole in different conditions.a |
We further compared the catalytic performance of various carbon materials,including the most commonly used active carbon (AC) and mesoporous graphitic carbon nitride (mpg-C3N4). With the same palladium loading content of 3%,the other two catalysts of Pd@AC and Pd@mpg-C3N4were prepared and utilized in the present reaction,with the catalytic results recorded in Table 1 (entries 8 and 9). It was found that Pd@AC demonstrated far lower activity than Pd/carbon nitride catalysts,with a conversion of only 39% in contrast to 75% for Pd@mpg-C3N4 and 96% fo Pd@CN0.132. The incorporation of nitrogen into the carbon matrix improved the percentage of Pd0 . The Pd3d X-ray photoelectron spectroscopies (XPS) illustrated that the content of Pd0 was 71% for Pd@ CN0.132 [39],70% for Pd@ C3N4[40],and 55.5% for Pd@AC [46]. We believed that the superior activity of Pd@ C3N4and Pd@ CN0.132can be attributed to the increased proportions of Pd0 ,which was reported as the active site for the activation of H2[47]. Such results were also obtained in the selective hydrogenation of the C55N bond in quinoline [45]. Furthermore,the content of nitrogen in the carbon materials also led to differences in catalytic activity (entries 6 and 8). The reason may arise from the fact that the surface of mpg-C3N4 is more electronegative than that of CN0.132. The higher electronegativity will strengthen the interaction between the catalyst and the protonated indoline and hinder the desorption step,with the comprehensive result of relatively slow reaction rate.
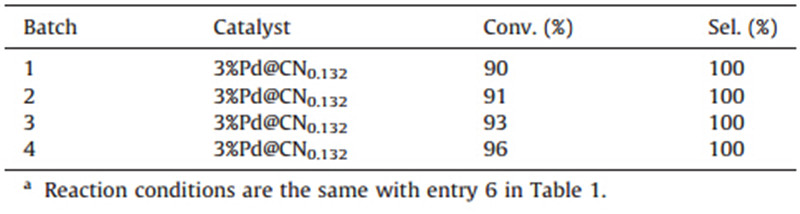
To verify the repeatability of the preparative procedure, catalysts from different batches were compared in parallel in this hydrogenation reaction with the results listed in Table 2. It is clear that all the catalysts from all four batches possess selectivity of 100% and conversion of more than 90%,which indicates good repeatability of the preparative procedure for the catalyst.
| Table 2 Catalytic results of various catalysts prepared from different batches.a |
In the present heterogeneous hydrogenation process,the addition of acid was necessary as shown in Table 3. If no acid was added into the reaction,a poor conversion of only 22% after 3 h was obtained (entry 1). Generally,the addition of acid will promote the reaction,but the degree of promotion is evidently different for various acids. Phosphoric acid possesses better performance than sulfuric acid,formic acid,and acetic acid (entries 2-5). However,p-toluenesulfonic acid (p-TSA) cannot promote the reaction (entries 1 and 6). Although the function of acid is generally assumed to provide protons to protonate the most nucleophilic C-3 position of indole and transfer the substrate from organic phase to aqueous phase [48],the anion of acid indeed plays a synergistic role in the process according to the above results. We also added different kinds of bases in this reaction to further understand the reaction pathway. As an inorganic base,Na2CO3 was chosen for this reaction system.
| Table 3 Influence of different additives on the hydrogenation of indole.a |
Disappointingly,the conversion was even lower than in reactions without any additive (entry 7). Addition of triethylamine also resulted in a low conversion of 9% (entry 8). These results further support theories on the function of acid.
Furthermore,we want to know if the conversion can reach completeness under higher hydrogen pressure. Disappointedly, the conversion with H3PO4as additive at 10 bar H2is almost the same with that obtained at atmospheric H2 (entries 2 and 10), while the conversion can slightly increase with H2 pressure increasing from 1 bar to 30 bar without the acid additive (entries 1 and 9). This demonstrates that H2pressure has little effect on the present reaction.
Based on to the above experimental results,we speculate the reaction mechanism as shown in Fig. 1. Firstly,a proton produced from the acid additive attacks the most nucleophilic C-3 position of indole to form iminium ion and the aromaticity of indole is temporarily destroyed,then the iminium ion quickly enters into the aqueous phase because of its strong hydrophilicity. This step is crucial to catalytic performance,and the further nucleophilic attack by another indole molecule is prevented because of phase separation and the solvation shell,so the polymerization reaction is eliminated,and the selectivity of indoline is enhanced. Subsequently,the iminium ion is hydrogenated by the dissociated hydrogen atoms attached on the surface of Pd nanoparticles to form indoline. Finally,the product of indoline leaves from the catalyst surface and enters into the organic phase.

|
Download:
|
| Fig. 1. Possible reaction pathway for hydrogenation of indole in water. | |
In the end,the molar ratio of indole to phosphoric acid was optimized with the results illustrated in Table 4. It is evident that the molar ratio of indole to phosphoric acid produces almost no change on the selectivity,but significant effects on the conversion. The conversion of indole increases from 44% to 96% (entries 3-6) when the molar ratio of indole to phosphoric acid ranges from 1:0.3 to 1:2.0. According to the proposed pathway,the proton is considered to play a ‘‘carrier’’ role in the catalytic cycles. Therefore, in the dilute region of acid,the conversion is proportional to the concentration of H+ . When the molar ratio of substrate to phosphoric acid reaches 1:1.2,the ‘‘carrier’’ maybe reaches saturation. If excess acid is added into the mixture,the excess H+ will occupy the surface of the catalyst and prohibit the adsorption of iminium ion,thus the conversion decreases with excess acid.
| Table 4 Influence of mole ratio of substrate to phosphate acid on the conversion and selectivity.a |
In summary,a new heterogeneous hydrogenation protocol for unprotected indole has been developed. In the presence of H3PO4, the reaction is carried out using Pd/CN0.132as catalyst at near room temperature and atmospheric H2in water with high selectivity and satisfactory conversion. The process is in accordance with the principles of green chemistry and provides an efficient methodology for chemoselective hydrogenation of unprotected indole to indoline.
AcknowledgmentsFinancial support from the Joint Petroleum and Petrochemical Funds of the National Natural Science Foundation of China and China National Petroleum Corporation (No. U1162124),the Zhejiang Provincial Natural Science Foundation for Distinguished Young Scholars of China (No. LR13B030001),the Specialized Research Fund for the Doctoral Program of Higher Education (No. J20130060),the National Natural Science Foundation of China (No. 21206085),Excellent Middle-aged and Young Scientists Research Award Foundation of Shandong Province (No. BS2011CL023),the Fundamental Research Funds for the Central Universities,the Program for Zhejiang Leading Team of S&T Innovation,and the Partner Group Program of Zhejiang University and the Max-Planck Society are greatly appreciated.
| [1] | R. Natesh, S.L.U. Schwager, H.R. Evans, E.D. Sturrock, K.R. Acharya, Structural details on the binding of antihypertensive drugs captopril and enalaprilat to human testicular angiotensin I-converting enzyme, Biochemistry 43 (2004) 8718-8724. |
| [2] | A.B. Dounay, L.E. Overman, A.D. Wrobleski, Sequential catalytic asymmetric Heckiminium ion cyclization: enantioselective total synthesis of the strychnos alkaloid minfiensine, J. Am. Chem. Soc. 127 (2005) 10186-10187. |
| [3] | N. Gruenfeld, J.L. Stanton, A.M. Yuan, et al., Huebner, Angiotensin converting enzyme inhibitors: 1-glutarylindoline-2-carboxylic acid derivatives, J. Med. Chem. 26 (1983) 1227-1282. |
| [4] | S. Anas, H.B. Kagan, Routes toward enantiopure 2-substituted indolines: an overview, Tetrahedron: Asymmetry 20 (2009) 2193-2199. |
| [5] | D.Y. Liu, G.W. Zhao, L. Xiang, Diverse strategies for the synthesis of the indoline scaffold, Eur. J. Org. Chem. 21 (2010) 3975-3984. |
| [6] | A. Smith, J.H.P. Utley, The catalytic hydrogenation of indoles, J. Chem. Soc. Chem. Commun. (1965) 427-428. |
| [7] | H. Ishii, K. Murakami, E. Sakurada, K. Hosoya, Y. Murakami, Polymerisation of indole. Part 2. A new indole trimer, J. Chem. Soc. Perkin Trans. 1 (1988) 2377-2385. |
| [8] | C.W. Bird, Heteroaromaticity. 5. A unified aromaticity index, Tetrahedron 48 (1992) 335-340. |
| [9] | Y.G. Zhou, Asymmetric hydrogenation of heteroaromatic compounds, Acc. Chem. Res. 40 (2007) 1357-1366. |
| [10] | G.W. Gribble, J.H. Hoffman, Reaction of sodium borohydride in acidic media; VI. Reduction of indoles with cyanoborohydride in acetic acid, Synthesis 12 (1977) 859-860. |
| [11] | D.M. Ketcha, B.A. Lieurance, The reduction of N-(phenylsulfonyl) indoles without sodium cyanoborohydride in trifluoroacetic acid, Tetrahedron Lett. 30 (1989) 6833-6836. |
| [12] | A. Srikrishana, T.J. Reddy, R. Viswajanani, Reductioxi of quinolines to 1,2,3,4-tetrahydro derivatives employing a combination of NaCNBH3, and BF3-OEt2, Tetrahedron 52 (1996) 1631-1636. |
| [13] | J.G. Berger, A rapid convenient reduction of indoles to indulines and of tetrahydrocarbazoles to hexahydrocarbazoles by trimethylamine/borane, Synthesis 7 (1974) 508-510. |
| [14] | A.E. Lanzilotti, R. Littell, W.J. Fanshawe, T.C. McKenzie, F.M. Lovell, Stereoselective reduction of some indoles with triethylsilane-trifluoroacetic acid, J. Org. Chem. 44 (1979) 4809-4813. |
| [15] | M.X. Tan, Y.G. Zhang, An efficient metal-free reduction using diphenylsilane with (tris-perfluorophenyl) borane as catalyst, Tetrahedron Lett. 50 (2009) 4912-4915. |
| [16] | A.M. Voutchkova, D. Gnanamgari, C.E. Jakobsche, et al., Selective partial reduction of quinolines: hydrosilylation vs. transfer hydrogenation, J. Organomet. Chem. 693 (2008) 1815-1821. |
| [17] | Y. Kikugawa, M. Kashimura, Catalytic transfer hydrogenation of indoles to indolines in formic acid, Synthesis 9 (1982) 785-787. |
| [18] | S. Chandrasekhar, D. Basu, C.R. Reddy, Palladium-catalyzed reduction of N-(tert-butoxycarbonyl) indoles by polymethylhydrosiloxane, Synthesis (2007) 1509-1512. |
| [19] | G.W. Gribble, P.D. Lord, J. Skotnicki, et al., Reactions of sodium borohydride in acidic media. I. Reduction of indoles and alkylation of aromatic amines with carboxylic acids, J. Am. Chem. Soc. 96 (1974) 7812-7814. |
| [20] | B. Robinson, The reduction of indoles and related compounds, Chem. Rev. 69 (1969) 785-797. |
| [21] | P. Barbaro, C. Bianchini, A. Meli, M. Moreno, F. Vizza, Hydrogenation of indole by phosphine-modified rhodium and ruthenium catalysts, Organometallics 21 (2002) 1430-1437. |
| [22] | A.F. Borowski, S. Sabo-Etienne, B. Donnadieu, B. Chaudret, Reactivity of the bis(dihydrogen) complex [RuH2(h2-H2)2(PCy3)2] toward N-heteroaromatic compounds. Regioselective hydrogenation of acridine to 1,2,3,4,5,6,7,8-octahydroacridine, Organometallics 22 (2003) 1630-1637. |
| [23] | R. Kuwano, M. Kashiwabara, Ruthenium-catalyzed asymmetric hydrogenation of N-Boc-indoles, Org. Lett. 8 (2006) 2653-2655. |
| [24] | R. Kuwano, K. Kaneda, T. Ito, K. Sato, T. Kurokawa, Highly enantioselective synthesis of chiral 3-substituted indolines by catalytic asymmetric hydrogenation of indoles, Org. Lett. 6 (2004) 2213-2215. |
| [25] | R. Kuwano, K. Sato, T. Kurokawa, D. Karube, Y. Ito, Catalytic asymmetric hydrogenation of heteroaromatic compounds, indoles, J. Am. Chem. Soc. 122 (2000) 7614-7615. |
| [26] | A. Baeza, A. Pfaltz, Iridium-catalyzed asymmetric hydrogenation of N-protected indoles, Chem. Eur. J. 16 (2010) 2036-2039. |
| [27] | D.S. Wang, Q.A. Chen, W. Li, et al., Pd-catalyzed asymmetric hydrogenation of unprotected indoles activated by brønsted acids, J. Am. Chem. Soc. 132 (2010) 8909-8911. |
| [28] | D.S. Wang, J. Tang, Y.G. Zhou, et al., Dehydration triggered asymmetric hydrogenation of 3-(α-hydroxyalkyl) indoles, Chem. Sci. 2 (2011) 803-806. |
| [29] | G.Y. Fan, W.J. Huang, Synthesis of ruthenium/reduced graphene oxide composites and application for the selective hydrogenation of halonitroaromatics, Chin. Chem. Lett. 25 (2014) 359-363. |
| [30] | J. Zhao, L. Ma, X.L. Xu, F. Feng, X.N. Li, Synthesis of carbon-supported Pd/SnO2 catalyst for highly selective hydrogenation of 2,4-difluoronitrobenzene, Chin. Chem. Lett. 25 (2014) 1137-1140. |
| [31] | X.L. Zhao, K.F. Yang, Y.P. Zhang, J. Zhu, L.W. Xu, Sevelamer as an efficient and reusable heterogeneous catalyst for the Knoevenagel reaction in water, Chin. Chem. Lett. 25 (2014) 1141-1144. |
| [32] | J.E. Shaw, P.R. Stapp, Regiospecific hydrogenation of quinolines and indoles in the heterocyclic ring, J. Heterocycl. Chem. 24 (1987) 1477-1483. |
| [33] | A. Kulkarni, W.H. Zhou, B. Török, Heterogeneous catalytic hydrogenation of unprotected indoles in water: a green solution to a long-standing challenge, Org. Lett. 13 (2011) 5124-5127. |
| [34] | H. Cho, F. Török, B. Török, Selective reduction of condensed N-heterocycles using water as a solvent and a hydrogen source, Org. Biomol. Chem. 11 (2013) 1209-1215. |
| [35] | M.F. Fang, N. Machalaba, R.A. Sanchez-Delgado, Hydrogenation of arenes and Nheteroaromatic compounds over ruthenium nanoparticles on poly(4-vinylpyridine): a versatile catalyst operating by a substrate-dependent dual site mechanism, Dalton Trans. 40 (2011) 10621-10632. |
| [36] | D. Clarisse, B. Fenet, F. Fache, Hexafluoroisopropanol: a powerful solvent for the hydrogenation of indole derivatives. Selective access to tetrahydroindoles or cisfused octahydroindoles, Org. Biomol. Chem. 10 (2012) 6587-6594. |
| [37] | N.A. Beckers, S. Huynh, X.J. Zhang, E.J. Luber, J.M. Buriak, Screening of heterogeneous multimetallic nanoparticle catalysts supported on metal oxides for mono-, poly-, and heteroaromatic hydrogenation activity, ACS Catal. 2 (2012) 1524-1534. |
| [38] | S. Coulton, T.L. Gilchrist, K. Graham, Catalytic hydrogenation of N-t-butoxycarbonylindoles, Tetrahedron 53 (1997) 791-798. |
| [39] | X. Xu, Y. Li, Y.T. Gong, et al., Synthesis of palladium nanoparticles supported on mesoporous N-doped carbon and their catalytic ability for biofuel upgrade, J. Am. Chem. Soc. 134 (2012) 16987-16990. |
| [40] | Y. Wang, J. Yao, H.R. Li, D.S. Su, M. Antonietti, Highly selective hydrogenation of phenol and derivatives over a Pd@carbon nitride catalyst in aqueous media, J. Am. Chem. Soc. 133 (2011) 2362-2365. |
| [41] | Y. Wang, Y. Di, M. Antonietti, et al., Excellent visible-light photocatalysis of fluorinated polymeric carbon nitride solids, Chem. Mater. 22 (2010) 5119-5121. |
| [42] | Y. Li, X. Xu, P.F. Zhang, et al., Highly selective Pd@mpg-C3N4 catalyst for phenol hydrogenation in aqueous phase, RSC Adv. 3 (2013) 10973-10982. |
| [43] | D.S. Deng, Y. Yang, Y.T. Gong, et al., Palladium nanoparticles supported on mpg-C3N4 as active catalyst for semihydrogenation of phenylacetylene under mild conditions, Green Chem. 15 (2013) 2525-2531. |
| [44] | Y. Li, Y.T. Gong, X. Xu, et al., A practical and benign synthesis of amines through Pd@mpg-C3N4 catalyzed reduction of nitriles, Catal. Commun. 28 (2012) 9-12. |
| [45] | Y.T. Gong, P.F. Zhang, X. Xu, et al., A novel catalyst Pd@ompg-C3N4 for highly chemoselective hydrogenation of quinoline under mild conditions, J. Catal. 297 (2013) 272-280. |
| [46] | H.Y. Jin, T.Y. Xiong, Y. Li, et al., Improved electrocatalytic activity for ethanol oxidation by Pd@N-doped carbon from biomass, Chem. Commun. 50 (2014) 12637-12640. |
| [47] | V.Z. Radkevich, T.L. Senko, K. Wilson, et al., The influence of surface functionalization of activated carbon on palladium dispersion and catalytic activity in hydrogen oxidation, Appl. Catal. A 335 (2008) 241-251. |
| [48] | R.L. Hinman, E.B. Whipple, The protonation of indoles: position of protonation, J. Am. Chem. Soc. 84 (1962) 2534-2539." |