The pyridine ring is a core structure presenting in a number of pharmaceuticals and natural products [1, 2, 3]. Pyridine derivatives and their nucleoside analogs showed strong cytotoxicity against several human cancer cell [4],antimycobacterial activities [5],and have been used for enrichment of cereals [6],and for regulation of arterial pressure [7] and cholesterol levels in blood [8]. In addition, some of polysubstituted pyridines are used as non-linear optical materials [9],the ability of chelating agent to complex various metal ions [10],Luminescent agents and building block for supramolecular chemistry [11] and has fluorescent liquid crystals [12]. Numerous methods are reported for the construction of pyridines by varying substitution pattern around the ring. A very common approach for the synthesis of pyridines is the condensation of 1,5-diketone with ammonia followed by nitric acid oxidation [13]. The reaction of β-aminoenones with malononitrile furnishing pyridones is known [14]. The reaction of α,β-unsaturated ketones and malononitrile in the presence of ammonium acetate has been reported [15]. Thiopyridines such as 2-amino-3,5-dicyano-6-sulfanylpyridines and corresponding 1,4-dihydropyridines have been prepared [16] from the reaction of an aldehyde,malononitrile,and a thiol. The synthesis of 2-amino-6-(arylthio)-4-arylpyridine-3, 5-dicarbonitriles via a three-component condensation reaction with various aldehydes,arylthiols and malononitrile is reported using catalytic ZrOCl2·8H2O/NaNH2 in [bmim]BF4 under ultrasound irradiation at room temperature [17]. A series of 2-pt-olylthiopyridine derivatives was directly synthesized via threecomponent reactions of chalcones,malononitrile,and 4-methylbenzenethiol catalyzed by Et3N in DMF under microwave irradiation [18]. Cyclotrimerization of a nitrile and two alkynes in the presence of cobalt (II) catalysts [19] is an alternative approach for the construction of highly functionalized pyridines. We report here a highly efficient procedure for the preparation of 4,6-diaryl-2-(arylthio)nicotinonitriles via one-pot four component reaction between aromatic aldehydes,acetophenone,malononitrile and thiols using non-ionic surfactant catalyst in water (Scheme 1). Triton X-100 (TR) is one of the most commonly used detergents in biochemistry as solublizer with a wide range of applications to biological systems [20]. Solublization of lipid membranes triggered by Triton X-100 is a well-described phenomenon. It is also used as an emulsifier,and complexing agent in both aqueous and non-aqueous media. Non-ionic surfactants have the tendency to adsorb at interfaces and to form micelles beyond their critical micelle concentration (CMC) similar to the ionic surfactants [21]. However; the advantage of non-ionic surfactants (Triton X-100) is the absence of the electrical double layer as formed by the ionic surfactants. Consequently,non-ionic surfactants are desirable model adsorbents for interfacial processes. Therefore,we decided to exploit these properties of non-ionic surfactant for organic reaction.
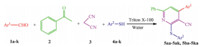
|
Download:
|
| Scheme 1. Synthesis of 2-(arylthio)nicotinonitriles in Triton X-100 aqueous micellar media. | |
Aldehydes were distilled before use and all chemicals used were reagent grade and were used as received without further purification. Melting points were determined using a Linkman HF591 heating stage,used in conjunction with a TC92 controller, and re uncorrected. NMR spectra were recorded using either a Brucker DRX500 machine at room temperature. 1H NMR and 13C NMR spectra were measured using DMSO-d6 as solvent and chemical shifts were measured relatives to residual solvent or CFCl3 as an internal standard for 19FNMR and are expressed in δ. Mass spectra were obtained using a Micro Mass LCT machine in ES or EI mode. Infrared spectra were measured on a Perkin Elmer Paragon 100 FT-IR spectrometer. All Analytical thin layer chromatography was performed with E. Merck silica gel 60F254 aluminum sheets and was visualized with UV light.
A mixture of benzaldehyde (1 mmol),acetophenone (1 mmol), malononitrile (1 mmol),with thiolphenol (1 mmol) were taken in a mixture of Triton X-100 (5 mol%) and water (2 mL) in a round bottomed flask. The resulting mixture was vigorously stirred at room temperature until completion of the reaction as monitored by thin-layer chromatography (TLC). After completion,the reaction mixture was poured onto crushed ice (70 g) with vigorous stirring. The precipitate obtained was filtered,washed with water, dried,and purified by column chromatography on silica gel (60- 120 mesh,ethyl acetate/hexane,1:3) to afford pure products. Structures of the all the products were confirmed by analytical and spectral data. Some selected data are listed below,and others please see the Supporting information.
4,6-Diphenyl-2-(phenylthio)nicotinonitrile (5aa): White powder; 335 mg (92%); mp 235 ℃; IR (KBr,cm-1): ν 3105,2212,1578, 1523,1453,1382,1092,973,866,745,729; 1H NMR (DMSO-d6, 500 MHz): δ 8.69 (d,2H,J = 7.5 Hz,ArH),7.97 (d,2H,J = 8.0 Hz, ArH),7.84-7.65 (m,3H,ArH),7.54-7.46 (m,3H,ArH),7.40-7.32 (m,2H,ArH),7.30-7.25 (m,4H,ArH); 13C NMR (DMSO-d6, 125 MHz): δ 180.21,160.21,159.23,139.54,138.25,137.65, 136.58,135.74,134.65,133.85,132.11,131.11,130.14,129.44, 128.33,126.47,125.75,124.12,123.22,122.12,121.41,117.14, 112.76,101.34; MS (EI) m/z (%): 364 (M+,20),255 (85); HRMS (EI) Found: M+,364.1010. C24H16N2S requires M+,364.1011; Anal. Calcd. for C24H16N2S: C,79.09; H,4.42; N,7.69; S,8.80; Found: C, 79.12; H,4.38; N,7.61; S,8.72. 2-((4-Fluorophenyl)thio)-4,6-diphenylnicotinonitrile (5ad): White powder; 363 mg (95%); mp 247 ℃; IR (KBr,cm-1): ν 3095,2199,1625,1510,1451,810,735,726; 1H NMR (DMSO-d6, 500 MHz): δ 8.66 (d,2H,J = 8.0 Hz,ArH),8.09 (d,2H,J = 8.0 Hz, ArH),7.77-7.64 (m,3H,ArH),7.60-7.49 (m,3H,ArH),7.45-7.21 (m,2H,ArH),7.12-7.02 (m,3H,ArH); 13C NMR (DMSO-d6, 125 MHz): δ 180.05,160.40,159.64,140.05,139.92,138.04,135.07 (d,1JCF = 253.58 Hz),136.05,135.14,133.90,132.17,131.74, 130.12,129.32,128.45,126.47,125.24,124.89,123.33,122.12, 122.94,120.47,116.74,111.74,100.15; 19F NMR (DMSO-d6, 470 MHz): -71.82; MS (EI),m/z (%): 382 (M+,15),292 (65); HRMS (EI) Found: M+,382.0912. C24H15FN2S requires M+, 382.0489; Anal Calcd. for C24H15FN2S: C,75.37; H,3.95; N,7.32; S,8.38. Found: C,75.41; H,3.92; N,7.43; S,8.31.
2-((2-Fluorophenyl)thio)-4,6-diphenylnicotinonitrile (5ae): White powder; 344 mg (90%); mp 251 ℃; IR (KBr,cm-1): n 3089,2201,1635,1525,1485,820,725,735; 1H NMR (DMSO-d6, 500 MHz): δ 8.12 (d,2H,J = 8.0 Hz,ArH),8.02 (d,2H,J = 8.0 Hz, ArH),7.41-7.39 (m,3H,ArH),7.35-7.30 (m,3H,ArH),7.25-7.21 (m,2H,ArH),7.19-7.10 (m,3H,ArH); 13C NMR (DMSO-d6, 125 MHz): δ 180.14,160.48,159.54,140.35,139.32,138.34,136.65 (d,1JCF = 252.18 Hz),135.15,134.18,133.25,132.17,131.74, 130.56,128.32,128.35,126.69,125.25,124.42,123.22,122.54, 122.68,120.57,117.54,115.41,101.21; 19F NMR (DMSO-d6, 470 MHz): -69.81; MS (EI),m/z (%): 382 (M+,15),292 (65); HRMS (EI) Found: M+,382.0602. C24H15FN2S requires M+, 382.0903; Anal Calcd. for C24H15FN2S: C,75.37; H,3.95; N,7.32; S,8.38. Found: C,75.41; H,3.89; N,7.43; S,8.21.
4,6-Diphenyl-2-((4-(trifluoromethyl)phenyl)thio)nicotinonitrile (5aj): White powder; 345 mg (80%); mp 246 ℃; IR (KBr, cm-1): ν 3079,2989,2239,1641,1565,1454,1105,845,717,715; 1H NMR (DMSO-d6,500 MHz): δ 8.72 (d,2H,J = 8.0 Hz,ArH),8.12 (d,2H,J = 8.0 Hz,ArH),8.10-7.98 (m,2H,ArH),7.93 (d,1H, J = 8.0 Hz,ArH),7.84-7.52 (m,3H,ArH),7.50-7.31 (m,2H,ArH), 4.16 (s,3H,OCH3); 13C NMR (DMSO-d6,125 MHz): δ 180.26, 160.45,159.12,140.14,139.14,138.14,136.58,135.17,134.02, 134.63,134.96,132.50,132.90,131.18,130.17,128.31,126.45, 126.89 (q,J = 283.5 Hz,CF3),125.45,122.43,121.48,120.09. 116.25,115.54,103.34; 19F NMR (DMSO-d6,470 MHz): -43.25; MS (EI),m/z (%): 432 (M+,5),286 (65); HRMS (EI) Found: M+, 432.0912. C24H15F3N2S requires M+,439.0901; Anal Calcd. for C24H15F3N2S: C,69.43; H,3.50; N,6.48; S,7.41. Found: C,69.52; H, 3.41; N,6.51; S,7.36.
2-(Naphthalen-2-ylthio)-4,6-diphenylnicotinonitrile (5ak): White powder; 393 mg (95%); mp 253 ℃; IR (KBr,cm-1): ν 3102,3054,2247,1643,1558,1448,827,728,713; 1H NMR (DMSO-d6,500 MHz): δ 8.89 (2H,d,J = 7.5 Hz,ArH),8.45 (d,2H, J = 7.5 Hz,ArH),7.89-7.78 (m,2H,ArH),7.70 (d,1H,J = 8.0 Hz, ArH),7.65-7.55 (m,4H,ArH),7.50-7.42 (m,2H,ArH),7.40-7.25 (m,3H,ArH); 7.20-7.08 (m,2H,ArH); 13C NMR (DMSO-d6, 125 MHz): δ 180.65,161.58,159.45,140.12,139.23,139.92, 138.88,136.65,134.88,134.93,133.12,133.85,132.65,130.01, 130.19,129.41,127.30,126.14,124.89,122.43,121.65,120.89, 117.32,116.54,100.95,100.05; 19F NMR (DMSO-d6,470 MHz): -74.25; MS (EI),m/z (%): 414 (M+,7),285 (85); HRMS (EI) Found: M+,414.1209. C28H18N2S requires M+,414.1220; Anal Calcd. for C28H18N2S: C,81.13; H,4.38; N,6.76; S,7.74. Found: C,81.21; H, 4.45; N,6.85; S,7.85.
6-(4-Fluorophenyl)-4-phenyl-2-(phenylthio)nicotinonitrile (5ha): White powder; 343 mg (90%); mp 250 ℃; IR (KBr,cm-1): n 3082,2212,1668,1553,1452,821,714,708; 1H NMR (DMSO-d6, 500 MHz): δ 8.81 (q,1H,J = 7.4 Hz,4JHF = 2.0 Hz,ArH),7.78 (s,2H, ArH),7.56 (d,2H,J = 7.5 Hz,ArH),7.48-7.38 (m,4H,ArH),7.34- 7.24 (m,3H,ArH),7.20-7.03 (m,3H,ArH); 13C NMR (DMSO-d6, 125 MHz): δ 180.19,160.32,159.62,140.14,139.19,138.36, 137.12,136.01,135.24 (d,1JCF = 250.41 Hz),134.12,133.10, 132.15,131.21,130.05,129.03,124.36,123.09,122.43,121.14, 120.08,120.45,120.89. 113.19,101.34; 19F NMR (DMSO-d6, 470 MHz): -73.44; MS (EI),m/z (%): 382 (M+,11),305 (51); HRMS (EI) Found: M+,382.0910. C24H15FN2S requires M+, 382.0911; Anal Calcd. for C24H15FN2S: C,75.37; H,3.95; N,7.32; S,8.38. Found: C,75.56; H,4.02; N,7.46; S,8.42.
6-(Naphthalen-2-yl)-4-phenyl-2-(phenylthio)nicotinonitrile (5ka): White powder; 372 mg (90%); mp 268 ℃; IR (KBr,cm-1): n 3089,2241,1661,1525,1450,826,729,716 cm-1; 1H NMR (DMSO-d6,500 MHz): δ 8.91 (s,1 H,ArH),8.56 (d,2H,J = 7.5 Hz, ArH),8.15 (dd,2H,J = 7.5 Hz,J = 2.5 Hz,ArH),7.82 (d,2H,J = 7.5 Hz, ArH),7.71-7.56 (m,5H,ArH),7.46-7.30 (m,6H,ArH); 13C NMR (DMSO-d6,125 MHz): δ 182.32,160.23,159.86,141.21,140.69, 139.22,138.34,137.46,135.04,133.09,133.15,132.85,131.30, 128.02,126.63,125.32,123.39,123.78,122.13,121.65,121.97, 120.06,113.19,101.67; MS (EI),m/z (%): 414 (M+,29),337 (47); HRMS (EI) Found:M+,414.1210. C28H18N2S requiresM+,414.1301; Anal Calcd. for C28H18N2S: C,81.13; H,4.38; N,6.76; S,7.74. Found: C,81.09; H,4.29; N,6.78; S,7.82.
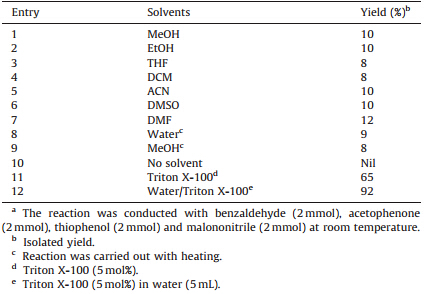
3. Results and discussionInitially,we screened various conditions for the one-pot fourcomponent reaction of benzaldehyde,acetophenone,thiophenol and malononitrile as a model reaction in methanol at room temperature. In polar solvents like methanol,ethanol,DMF and DMSO,product 5aa was obtained in higher yield. However,in nonpolar solvents such as dichloromethane,tetrahydrofuran,and acetonitrile,product 5aa were obtained in poor yield. When the reaction was carried out under solvent free condition at 100 ℃,we found no product. Also,when benzaldehyde,acetophenone, thiophenol and malononitrile were stirred at room temperature without water,using water as solvent and Triton X-100 (5 mol%) as a surfactant the yield of 5aa was improved (Table 1,entries 11,12). However,it was found that the amount of Triton X-100 (C14H22(C2H4O)n) where n = 9-10,influenced the yield of 5aa.
| Table 1 Effect of solvents on synthesis of 4,6-diphenyl-2-(phenylthio)nicotinonitrile.a |
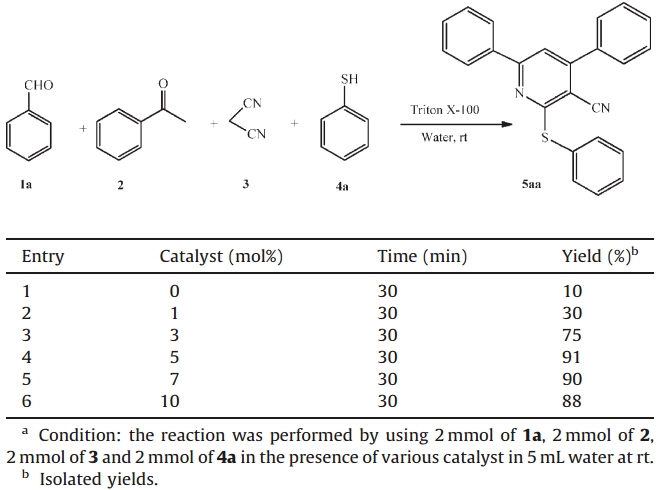
We studied the role of catalyst concentration on the model reaction 5aa.We have varied the catalyst concentration to 0,1,3,5, 7 and 10 mol%. The result revealed that,when the reaction was carried out in the presence of 0,1,3,7 and 10 mol% of catalyst it gave lower yields of product even after prolonged reaction time. At the same time when the concentration of the catalyst was 5 mol%, we got an excellent yield of product in a short span. Even after increasing the catalyst concentration above 5 mol% the yield of the products did not improve. So it is established that the 5 mol% of catalyst is sufficient to catalyst and bring it to completion. The results are listed in Table 2.
| Table 2 One-pot synthesis of 4,6-diphenyl-2-(phenylthio)nicotinonitrile in presence of various catalyst concentration.a |
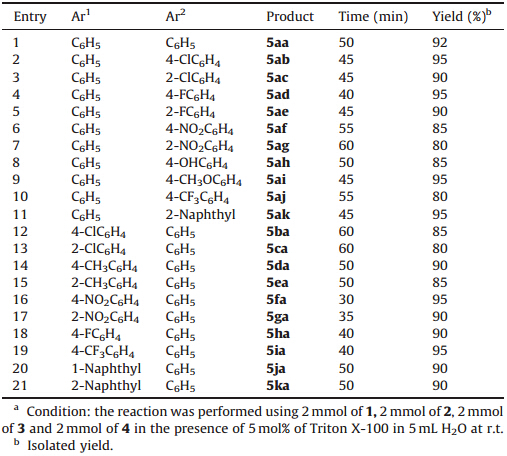
Under the optimized reaction conditions,a series of 4,6-diaryl- 2-(arylthio)nicotinonitriles 5 were synthesized (Table 3). First,the reactions of benzaldehyde 1a,acetophenone 2,malononitrile 3 and thiols 4a-k including either electron-withdrawing or electrondonating groups were investigated. It was found that all these products in good to excellent yields,(Table 3,entries 1-11),the rate of the reaction of thiol with electron-donating (entries 2-5,8, 9,Table 2) is faster than with electron rich aryl thiol (entries 6,7, Table 3). Then,we applied the optimal protocol to acetophenone 2, malononitrile 3,thiophenol 4a with a variable of substituted aryl aldehydes including either electron-withdrawing or electrondonating groups. Fortunately,satisfactory results were gained and rate of the reaction with electron-deficient aryl aldehyde (entries 16-21,Table 3) is faster than with electron-rich aryl aldehyde.
| Table 3 Triton X-100 aqueous micelles catalyzed one-pot synthesis of 4,6-diaryl-2- (arylthio)nicotinonitriles.a |
The catalytic effect of micellar Triton X-100 in these reactions can be explained as follows. Benzaldehyde,acetophenone, thiophenol and malononitrile,which react to produce nicotinonitrile derivatives,are hydrophobic molecules in aqueous media. In the micellar solution of Triton X-100,the hydrophobic moieties escape away from water molecules,which encircle the micelle core of Triton X-100. However,they are pushed by the water to go inside the hydrophobic core of the micelle droplets,where the reactions take place more easily. This explanation is schematically presented in Fig. 1.
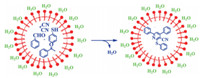
|
Download:
|
| Fig. 1. The function of micellar Triton X-100 droplets on the reactions in aqueous media. | |
We proposed the possible mechanism for the formation of 4,6- diaryl-2-(arylthio)nicotinonitriles in Triton X-100 aqueous micelles 5aa-5ak,5ba-5ka as shown in Scheme 2. The process represents a typical cascade reaction in which the aromatic aldehyde 1 first condense with acetophenone 2 to afford enone A. Then,enone A is attracted via Michael addition of malononitrile 3 to give the 2-(3-oxo-1-aryl-3-phenylpropyl)malononitrile B followed by the nucleophilic attract of thiol 4 to the cyano moiety to form the desired pentanimidothioate C. Enamine formation, cyclization,air oxidation of D and aromatization to afford 5aa- 5ak,5ba-5ka.
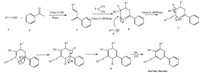
|
Download:
|
| Scheme 2. Plausible mechanism for the reaction of arylaldehydes,acetophenone,malononitrile with thiols. | |
In conclusion,non-ionic surfactant (Triton X-100) was found to be an efficient catalyst for the one-pot reaction of aromatic aldehyde,acetophenone,thiophenol and malononitrile to afford the corresponding nicotinonitriles in moderate to good yields. The main advantages of the present synthetic protocol are mild reaction,ecofriendly catalyst and easy work-up procedure. The reaction system can be successfully applied to a variety of aryl aldehyde and thiols to synthesize a wide variety of nicotinonitriles in good to excellent yields.
AcknowledgmentThis work was financially supported by the Payame Noor University,Iran.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.11.007.
| [1] | H. Mitsuya, R. Yarchoan, S. Broder, Molecular targets for AIDS therapy, Science 249 (1990) 1533-1544. |
| [2] | J.A. Montgomery, Approaches to antiviral chemotherapy, Antiviral Res. 12 (1989) 113-132. |
| [3] | X. Zhang, D. Li, X. Fan, et al., Ionic liquid-promoted multi-component reaction: novel and efficient preparation of pyrazolo[3, 4-b]pyridinone, pyrazolo[3,4-b]-quinolinone and their hybrids with pyrimidine nucleoside, Mol. Divers 14 (2010) 159-167. |
| [4] | O.M. Ahmed, M.A. Mohamed, R.R. Ahmed, S.A. Ahmed, Synthesis and anti-tumor activities of some new pyridines and pyrazolo[1, 5-a]pyrimidines, Eur. J. Med. Chem. 44 (2009) 3519-3523. |
| [5] | M.G. Mamolo, D. Zampieri, V. Falagiani, L. Vio, E. Banfi, Synthesis and antimycobacterial activity of 5-aryl-1-isonicotinoyl-3-(pyridin-2-yl)-4, 5-dihydro-1H-pyrazole derivatives, II Farmaco 56 (2001) 593-599. |
| [6] | C.O. Badgett, C.F. Woodward, Nicotinic acid, miscellaneous esters, J. Am. Chem. Soc. 69 (1947), 2907-2907. |
| [7] | R. Gupta, A. Jain, M. Jain, R. Joshi, ‘One pot’synthesis of 2-amino-3-cyano-4,6-diarylpyridines under ultrasonic irradiation and grindstone technology, Bull, Korean Chem. Soc. 31 (2010) 3180-3182. |
| [8] | G. Dorner, F.W. Fischer, On the modification of serum lipids and lipoproteins by the hexanicotinic acid ester of m-inositol, Arzenmittel. Forsch. 11 (1961) 110-113. |
| [9] | V.K. Indirapriyadharshini, P. Ramamurthy, V. Raghukumar, V.T. Ramakrishma, Spectral and photophysical properties of 1,6-naphthyridine derivatives: a new class of compounds for nonlinear optics, Spectrochim. Acta A 58 (2002) 1535-1543. |
| [10] | T.J. Meyer, Chemical approach to artificial photosynthesis, J. Acc. Chem. Res. 22 (1989) 163-170. |
| [11] | G.S. Hanan, D. Volmer, U.S. Schubert, et al., Coordination arrays: tetranuclear cobalt(II) complexes with [2 2]-Grid structure, Angew. Chem., Int. Ed. 36 (1997) 1842-1844. |
| [12] | A.I. Pavluchenko, V.F. Petrov, N.I. Simirnova, Liquid crystalline 2,5-disubstituted pyridine derivatives, Liq. Cryst. 19 (1995) 811-821. |
| [13] | (a) G.R. Pabst, J. Sauer, A new and simple ‘LEGO’ system for the synthesis of 2,6-oligopyridines, Tetrahedron Lett. 39 (1998) 6687-6690; (b) G.R. Pabst, O.C. Pfuller, J. Sauer, The new and simple ‘LEGO’system: synthesis and reactions of ruthenium (II) complexes, Tetrahedron Lett. 39 (1998) 8825-8828. |
| [14] | A. Alberola, L.A. Calvo, A.G. Ortega, R.M.C. Sanudo, P. Yustos, Regioselective synthesis of 2(1H)-pyridinones from b-aminoenones and malononitrile reaction mechanism, J. Org. Chem. 64 (1999) 9493-9498. |
| [15] | N.M. Evdokimov, I.V. Magedov, A.S. Kireev, A. Komienko, One-step, three-component synthesis of pyridines and 1,4-dihydropyridines with manifold medicinal utility, Org. Lett. 8 (2006) 899-902. |
| [16] | A.W. Fatland, B.E. Eaton, Cobalt-catalyzed alkyne-nitrile cyclotrimerization to form pyridines in aqueous solution, Org. Lett. 2 (2000) 3131-3133. |
| [17] | M.R. Poor Heravi, F. Fakhr, Ultrasound-promoted synthesis of 2-amino-6-(arylthio)-4-arylpyridine-3, 5-dicarbonitriles using ZrOCl2 8H2O as the catalyst in the ionic liquid [bmim]BF4 at room temperature, Tetrahedron Lett. 52 (2011) 6779-6782. |
| [18] | X.M. Rimaz, H. Mousavi, A one-pot strategy for regioselective synthesis of 6-aryl-3-oxo-2, 3-dihydropyridazine-4-carbohydrazides, Turk. J. Chem. 37 (2013) 252-261. |
| [19] | R.C.F. Jones, M.J. Smallridge, Annulation of imidazolines: synthesis of imidazo [1, 2-a] pyridones, Tetrahedron Lett. 29 (1988) 5005-5008. |
| [20] | M.N. Jones, Two-dimensional electrophoresis of membrane proteins using immobilized pH gradients, Int. J. Pharm. 177 (1999) 137-139. |
| [21] | X. Zeng, K. Osseo-Asare, Partitioning behavior of silica in the Triton X-100/ dextran/water aqueous biphasic system, J. Colloid Interface Sci. 272 (2004) 298-307. |