b Key Laboratory of Advanced Textile Materials and Manufacturing Technology, Ministry of Education of China, Hangzhou 310018, China;
c Zhejiang Longsheng Group Co., Ltd., Shangyu 312368, China
Silk with the properties of soft handle,elegant style and comfortable wearing is regarded as a luxurious clothing and decoration material [1, 2]. So far,acid dyes and reactive dyes have been considered as themost suitable synthetic dyes for silk although some problems still exist. For example,when silk fibers are dyed with conventional acid dyes,the exhaustion is only about 50% of the dye uptake on wool because of the lower amino end-group content in silk than in wool [3]. Besides,dyed silk has lower wet fastness properties [4, 5]. The poor wet fastness not only influences the appearance and the wearing properties of silk fabric,but also causes pollution fromdyeing andwashing during processing. Reactive dyes are considered to be comparatively ideal dyes for silk due to the formation of covalent bonds between dyes and silkmacromolecules, which offers the dyed silk relatively good wet fastness compared to acid dyes.However,the C-Oor C-Ncovalent bonds formedbetween dyes and silk mainly exist as ester or amide and are readily hydrolyzed under strong acidic or basic conditions [6],which leave the wet fastness problem unsolved. Besides,the hydrolysis of the reactive groups during dyeing processes results in a portion of dye unfixed on the fibers and pollutes the effluents [7, 8, 9].
Much effort has been devoted to improve the poorwet fastness of dyeing on silk. In one approach,it was noticed that there is a relatively high tyrosine residue content in silk protein (~6 mol%) and the residuemight be potential reactive sites for dyeing [10]. For example,the phenolic side chain in the tyrosine residues in silk could undergo coupling coloration with diazonium salts in an alkaline aqueous buffer solution at 0-10 ℃ to form an azobenzene chromophore linked to the protein through C-C covalent bonds [11, 12, 13, 14, 15],which is very stableunder acidic or basic conditions. This type of coloration is quite similar to the commercial process for azoic dyeing of cotton. However,the coupling coloration is difficult to cover full color spectrumdue to the immutable coupling components and the tyrosine residues. Besides,the phenolic side chain in the tyrosine residues could also undergo three-component Mannich-type reactions in the presence of formaldehyde and aromatic primary amine-containing functional compounds,which also forms stable and strong -CH2-NH- bridge connections between the tyrosine residues of silk and the functional moiety in an aqueous buffer solution (pH 5.5-6.5) at 37 ℃ [16, 17, 18]. If the functional moiety is a chromophore,the silk would be dyed by this Mannich-type dyeing method (Scheme 1). It can be predicted that the method is more energy-efficient,environmentally friendly and wet-colorfast than the acid dye and reactive dye methods.

|
Download:
|
| Scheme 1. The Mannich-type dyeing method on silk. | |
In this paper,an aromatic primary amine-containing acid dye AMODB suitable for Mannich dyeing has been synthesized. Its dyeing behaviors on silk were investigated under acidic and Mannich-type dyeing conditions. The washing fastness and the anti-stripping ability by DMF were also compared with a similar control acid dye (C.I. Acid Yellow 11) under acidic dyeing conditions,which confirms the formation of -CH2-NH- covalent bonding connections between dyes and silk fiber.
2. ExperimentalDegummed untreatedwhite,plain-woven silk fabric (43 g/m2) was used. C.I. Acid Yellow 11 was bought from Tianjin Xiangrui Dyes Co.,Ltd. (Tianjin,China). Other reagents were of analytic grade and obtained from commercial suppliers and used without further purification. 1H NMR spectra were recorded on a Varian INOVA 400 NMR Spectrometer with TMS as an internal standard in CDCl3. IR spectra were measured with an FT/IR-430 spectrophotometer. Mass spectra (MS) were determined by using an LCQ Fleet mass spectrometer. Ultraviolet-visible (UV-vis) absorption spectra were recorded on a Lambda 900 UV/vis spectrophotometer.
The synthetic route of the target dye AMODB is outlined in Scheme 2,which mainly contains two steps as follows:

|
Download:
|
| Scheme 2. The synthetic route of an aromatic primary amine-containing acid dye AMODB and the structure of the control dye C.I. Acid Yellow 11. | |
Synthesis of nitro-containing acid dyeMNODB by diazo coupling reaction: MNODB was synthesized by the conventional diazocoupling methods. p-Nitroaniline (6.9 g,0.05 mol)was dissolvedina mixture of concentrated hydrochloric acid (10 mL,37%) and water (50 ml). The solutionwas heated to 75 ℃ for about 30min and then cooled to 0-5 ℃. A 30% aqueous solution of sodium nitrite (3.52 g, 0.051 mol) was added to the p-nitroaniline solution and stirred for 30 min at 0-5 ℃. Further stirring at this temperature for 10 min resulted in a clear diazonium salt solution. The coupling reaction was carried out by adding the prepared diazonium salt solution to the coupling component (1-(4-sulfophenyl)-3-methyl-5-pyrazolone) solution (12.7 g,0.05mol) at 0-5 ℃,pH 8.5-9.0 for 3 h. ThepH value of the coupling liquor was controlled by adding sodium carbonate powder. The dye was salted out by adding 2.0 g of sodium chloride. Crude yield: 19.5 g (91.8%); FTIR (KBr,cm-1): 2927 (CH3), 1667 (C=O,pyrazolone),1502,1342 (NO2),1155,1043 (SO3Na); ESI MS (m/z,%): 402.1 (M-Na,100). The crude dye MNODB was then purified by the N,N-dimethylformamide/ether (3:20 v/v) purification method [19]. Yield: 15.6 g (73.4%).
Synthesis of aromatic primary amine-containing acid dye AMODB: The solution of nitro-containing acid dye MNODB (8.51 g,0.02 mol) in water (50 mL) was stirred and heated at 75 ℃ for about 30 min to dissolve MNODB. Then a solution of Na2S·9H2O (9.6 g,0.04mol),NaHCO3 (3.36 g,0.04 mol) in water (50 mL) was added dropwise into a solution of a nitro-containing acid dye MNODB,and the temperature was kept at 75 ℃ for about 4 h. After the acidification to pH 5-6 with dilute hydrochloric acid,the product was collected by filtration. The filter cake was air dried at room temperature. The pure dye AMODB was obtained by recrystallization in acetic acid. Yield: 5.8 g (73.4%); λmax: 447 nm; FTIR (ATR,cm-1): 3481,3361 (NH2), 2943 (CH3),1660 (C=O,pyrazolone),1162,1032 (SO3Na); 1H NMR (400 MHz,DMSO-d6): δ 13.63 (s,1H,=N-NH),7.90 (d,2H, Ar-H),7.65 (d,2H,Ar-H),7.36 (d,2H,Ar-H),6.66 (d,2H,Ar-H), 5.56 (s,2H,NH2),2.28 (s,3H,N=C-CH3); 13C NMR (125 M Hz, DMSO-d6): d 157.1,155.3,147.7,138.1,126.4,124.7,118.0, 116.5,114.3,112.3,99.5,14.4; ESI MS (m/z,%): 372.2 (M-Na, 100); element analysis: found (%): C,48.41,H,3.52,N,17.65; calcd. (%): C,48.61,H,3.57,N,17.71.
M1 (Mannich-type dyeing process for AMODB): Dye AMODB and a formaldehyde aqueous solutions were applied to dyeing silk fabric by the one-bath process using the Mannich reaction. A silk fabric sample (2 g) was dyed with AMODB at 3% owf, n (formaldehyde):n (AMODB) = 3:1,75:1 liquor-to-goods ratio, dyebath pH 5.5 and 30 ℃ for 10 h.
M2 (contrastive dyeing process for AMODB,without formaldehyde in comparison to M1): Only dye AMODB was applied to dyeing silk fabric by the one-bath process in the absence of formaldehyde in comparison to M1. A silk fabric sample (2 g) was dyed with AMODB at 3% owf,75:1 liquor-to-goods ratio,dyebath pH 5.5 and 30 ℃ for 10 h.
M3 (acidic dyeing process for C.I. Acid Yellow 11): A silk fabric sample (2 g) was dyed with C.I. Acid Yellow 11 at 3% owf,40:1 liquor-to-goods ratio,dyebath pH 5.5 and 90 ℃ for 90 min.
Soap-washed process: All dyed samples (M1,M2,M3) were rinsed with water,followed by soaping in a mixture of 1 g/L sodium carbonate and 2 g/L nonionic detergent using a liquor ratio of 50:1 at 60 ℃ for 30 min,rinsed with water and air-dried. Color stripping method with N,N-dimethylformamide (DMF): The colored silk fabric samples were immersed in DMF and stripped at 70 ℃ for 30 min. The remaining fabric samples were subsequently washed with plenty of water to remove DMF and then air-dried.
K/S spectra: The K/S values of the dyed silk fabric samples were evaluated at maximum absorption wavelength (λmax) using a UV/vis spectrophotometer. The K/S value is a function of the reflectance R,as expressed by Eq. (1). Each sample was read in four different areas and the average value was recorded.

Tensile strength tests: The tensile strength tests of the untreated and dyed silk fabric samples by the Mannich dyeing method were carried out according to ISO 13934.1-1999.
Color fastness tests: Washing and rubbing fastness tests of the silk fabric by coupling coloration were carried out according to ISO 105-C03:1989 and ISO 105-X12:1993,respectively.
3. Results and discussionThe Mannich reaction is a three-component condensation among an amine component,an enolizable carbonyl compound (donor),and a nonenolizable carbonyl compound (acceptor) to form a β-amino carbonyl compound,with the concomitant formation of both carbon-carbon and carbon-nitrogen bonds. For example,aniline compounds,phenol compounds and aldehydes can act as the amine component,donor and acceptor in Mannich reaction,respectively. The Mannich reaction is extendedly employed in many fields such as drug synthesis,agro chemicals synthesis and especially chemical modification of proteins.
It has been reported that the phenolic side chain in the tyrosine residues in proteins could undergo three-component Mannichtype reactions in the presence of formaldehyde and an anilinecontaining compound,which forms stable and strong -CH2-NH- bridge connections between the tyrosine residues and the moiety of the aniline-containing compound with high levels of selectivity under relatively mild conditions (pH 6.5,at about room temperature, 18 h) [16]. It was also found that the electron-rich anilines generally exhibit higher Mannich reactivity than the electrondeficient anilines. The results gave us a hint to develop a novel reactive dyeing method suitable for silk based on the Mannich reaction,in which the high content of tyrosine residues in silk act as donors,an electron-rich aniline dye as the amine component and formaldehyde as an acceptor.
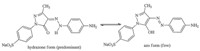
First of all,an aniline-containing dye AMODB was readily synthesized from reactant p-nitroaniline by successive diazotization, coupling reaction and reduction (Scheme 2). Then its molecular structure was characterized by FTIR,1H NMR,mass spectrometry and elemental analysis. It is known that azopyrazolone dyes predominantly exist in the hydrazone form over the azo form in the solid state and acidic solutions [20]. The FTIR and 1H NMR spectra of the dye provide some characteristic results to confirm this. The stretching vibration band of carbonyl appears at 1660 cm-1 in the FTIR spectrum. The hydrogen-bonded NH proton appears at 13.63 in the 1H NMR spectrum and its hydrogen integral is close to 1.0. The results suggest that AMODB nearly completely exists in the hydrazone form (Scheme 3). The existence of an imine group (-NH-) at the para position of aniline might make the aniline moiety electron-rich and exhibit high reactivity (dye fixation) in Mannich dyeing.
|
Download:
|
| Scheme 3. Changes between hydrazone and azo forms of phenylazopyrazolone dye AMODB. | |
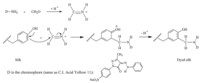
Dye AMODB was then applied to dyeing silk fabrics using the Mannich dyeing method and its dyeing process was named as M1. Its dyeing mechanism was described in Scheme 4. Although the Mannich-type reaction among aniline,formaldehyde and tyrosine residues in proteins was proven and characterized by NMR and MS analyses,the proof of Mannich-type reaction on silk is difficult to attain due to its polymeric properties such as large molecular weight,complex composition including eighteen kinds of amino acids,easy oxidation of dye chromophore during the hydrolysis in strong acidic or alkaline aqueous solutions at high temperatures. For example,the newly formed -N-C- bond in the Mannich-type reaction is not intense and characteristic in FTIR analysis. Besides, the NMR and elemental analyses need the separation and purification of the Mannich product,which is very complicated and difficult. The MS analysis also needs the hydrolysis of the dyed silk to achieve amino acid derivative containing dye chromophore. However,the harsh hydrolysis conditions often break azo chromophore in AMODB and compromise the characterization of the accurate structure of the dyed silk. In view of the difficulty in proving the Mannich-type reaction happened on silk,two basic indicators for proving the covalent bonding formation suitable for the reactive dyes,namely color depth (K/S) after dyeing and color stripping were utilized in Mannich dyeing. Moreover,a contrastive dyeing process M2 in the absence of formaldehyde in comparison to M1 was carried out in order to analyze the effect of formaldehyde in Mannich dyeing. Additionally,a control dye C.I. Acid Yellow 11 was used to dye silk fabrics with the traditional acidic dyeing method (M3) to compare the type of bonding with M1.
|
Download:
|
| Scheme 4. The Mannich-type dyeing mechanism of the aromatic primary amine-containing acid dye AMODB on silk. | |
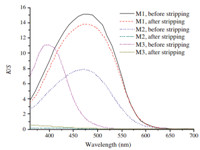
The soap-washed dyeing samples of M1,M2 and M3 were then color stripped by DMF to verify the type of binding between dyes and silk fibers. Fig. 1 shows the K/S spectra of M1,M2 and M3 before and after DMF stripping. It can be seen that the silk fabrics are successfully dyed by M1,M2and M3with different color depth. Both of the dyed silk fabric samples by M1 (before and after color stripping by DMF) showed the highest K/S value and the DK/S is lower relative to the K/S value before stripping,which is a strong evidence for covalent bond formation via the Mannich reaction. By comparison of the K/S spectra of M1 and M2 before and after color stripping,it is easily concluded that formaldehyde plays an important role in the Mannich dyeing. The absence of formaldehyde circumvents the Mannich reaction and the absorbed dyed on silk fabric is easy to stripped by DMF. As is well known that the acid dyes can combine with silk through ionic bonds,which is proved to be the case for dyed silk fabric sample with C.I. Acid Yellow 11. The great decline of K/S value ofM3after color stripping reveals the fact that the ionic bonds between dyes and silk fibers can be readily broken by polar solvent DMF. It can be predicted from the aforementioned results that the Mannich dyeing really happens among AMODB,formaldehyde and silk despite of the absence of direct spectral data.
|
Download:
|
| Fig. 1. The K/S spectra of dye AMODB and C.I. Acid Yellow 11 on silk fabrics by Mannich-type dyeing (M1),Mannich-type dyeing short of formaldehyde (M2) and acidic dyeing (M3) before and after color stripping. Dyeing conditions: (M1) AMODB,3% owf,n (formaldehyde):n (AMODB) = 3:1,75:1 liquor ratio,pH 5.5, 30 ℃,10 h; (M2) AMODB,3% owf,no formaldehyde,75:1 liquor ratio,pH 5.5, 30 ℃,10 h; (M3) C.I. Acid Yellow 11,3% owf,40:1 liquor ratio,pH 5.5,90 ℃, 90 min. | |
Table 1 shows the washing and rubbing fastness test results of dyed silk fabrics by the M1 and M3 processes. Clearly,the dyed sample by M1 exhibits same rubbing fastness and superior washing fastness in comparison with those of the dyed sample by M3. It is also an indirect piece of evidence for covalent bond formation between AMODB and silk fibers.
| Table 1 Color fastness properties of AMODB by M1 and C.I. Acid Yellow 11 by M3 on silk fabrics. |
Under the guidance of the finding that the tyrosine residues in proteins could undergo three-component Mannich-type reactions with formaldehyde and electron-rich aniline-containing compounds, which forms covalent bonding connections between the protein of interest and the anilines with high levels of selectivity under relatively mild conditions,an orange electron-rich anilinecontaining dye AMODB was designed and readily synthesized for Mannich-type dyeing on silk. It is proven that dye AMODB successfully reacts with silk fiber under mild dyeing conditions according to a Mannich-type dyeing mechanism by some indirect evidence such as higher color depth,better anti-stripping ability to DMF and better washing fastness in comparison to the control acid dye on silk fabric by the acidic dyeing method. The Mannich dyeing method can be developed into a new energy-efficient,environmentally friendly and reactive dyeing method suitable for silk, which might have much wider application prospects over the traditional reactive dyes.
AcknowledgmentsThe work was supported by the National Natural Science Foundation of China (Nos. 21106135 and 51173168),Zhejiang Provincial Key Innovation Team (No. 2010R50038),Zhejiang Provincial Top Key Academic Discipline of Chemical Engineering and Technology,and ‘‘521’’ Talent Project of Zhejiang Sci-Tech University.
| [1] | C. Solazzo, J.M. Dyer, S. Deb-Choudhury, Proteomic profiling of the photo-oxidation of silk fibroin: implications for historic tin-weighted silk, Photochem. Photobiol. 88 (2012) 1217-1226. |
| [2] | J. Shao, J. Liu, J. Zheng, C.M. Carr, X-ray photoelectron spectroscopic study of silk fibroin surface, Polym. Int. 51 (2002) 1479-1483. |
| [3] | Z. Cai, Fiber Chemistry and Physics, China Textile & Apparel Press, Beijing, 2004. |
| [4] | G. Mitra, S.K. Bhattacharya, P.K. Mazumdar, A review on chemical processing of silk, Colourage 56 (2009) 48-50, 52. |
| [5] | S. Shakra, S. Shakra, E.E. Allam, H.F. Mansour, Dyeing natural silk fabrics-part I: direct dyes, Am. Dyest. Rep. 88 (1999) 29-32. |
| [6] | J. He, Dye Chemistry, China Textile & Apparel Press, Beijing, 2009. |
| [7] | M. Inoue, F. Okada, A. Sakurai, M. Sakakibara, A new development of dyestuffs degradation system using ultrasound, Ultrason. Sonochem. 13 (2006) 313-320. |
| [8] | S.K. Kansal, M. Singh, D. Sud, Studies on photodegradation of two commercial dyes in aqueous phase using different photocatalysts, J. Hazard. Mater. 141 (2007) 581-590. |
| [9] | S. Vanhulle, E. Enand, M. Trovaslet, Overlap of laccases/cellobiose dehydrogenase activities during the decolourization of anthraquinonic dyes with close chemical structures by Pycnoporus strains, Enzym. Microb. Technol. 40 (2007) 1723-1731. |
| [10] | J.H. Zheng, J.Z. Shao, J.Q. Liu, Study on the distribution of tyrosine in silk fibroin, J. Text. Res. 22 (2001) 7-9. |
| [11] | H.M. Zhou, H.R. Wang, Chemical Modification of Protein, Qinghua University, Beijing, 1998. |
| [12] | W. Chen, Z. Wang, Z. Cui, et al., Study on coloration of silk based on coupling reaction with a diazonium compound, Fibers Polym. 15 (2014) 966-970. |
| [13] | J. Gavrilyuk, H. Ban, M. Nagano, W. Hakamata, C.F. Barbas, Formylbenzene diazonium hexafluorophosphate reagent for tyrosine-selective modification of proteins and the introduction of a bioorthogonal aldehyde, Bioconj. Chem. 23 (2012) 2321-2328. |
| [14] | J.M. Hooker, E.W. Kovacs, M.B. Francis, Interior surface modification of bacteriophage MS2, J. Am. Chem. Soc. 126 (2004) 3718-3719. |
| [15] | T.L. Schlick, Z. Ding, E.W. Kovacs, M.B. Francis, Dual-surface modification of the tobacco mosaic virus, J. Am. Chem. Soc. 127 (2005) 3718-3723. |
| [16] | N.S. Joshi, L.R. Whitaker, M.B. Francis, A three-component Mannich-type reaction for selective tyrosine bioconjugation, J. Am. Chem. Soc. 126 (2004) 15942-15943. |
| [17] | D.W. Romanini, M.B. Francis, Attachment of peptide building blocks to proteins through tyrosine bioconjugation, Bioconj. Chem. 19 (2008) 153-157. |
| [18] | J.M. McFarland, N.S. Joshi, M.B. Francis, Characterization of a three-component coupling reaction on proteins by isotopic labeling and nuclear magnetic resonance spectroscopy, J. Am. Chem. Soc. 130 (2008) 7639-7644. |
| [19] | J. Yang, Analysis and Anatomy of Dyes, Chemical Industry Press, Beijing, 1989. |
| [20] | K. Hunger, Industrial Dyes: Chemistry, Properties, Applications, Wiley-VCH Verlag GmbH & Co. KgaA, Weinheim, 2003.s |





