Nanostructured manganese dioxides (MnO2 ) represent a class of most studied transition metal oxides due to their unique crystalline structures and wide applications in molecular adsorption [1,2,3,4],catalysis [5,6],and electrochemical energy storage [7,8].
For example,using nanostructured MnO2 as active components of electrodes in energy storage devices has many advantages in terms of high specific energy capacity,low fabrication cost,and environmental benignity [9]. Similar to other transition metal oxides,MnO2 nanostructures can also be used as absorbents to remove organic contaminations from waste water and the adsorbed organic species can be burned through a combustion at relatively low temperature catalyzed by the MnO2 [1]. Previous studies have proven that performance of the MnO2 nanostructures in these applications strongly depends on morphology and crystallinity of the MnO2 nanostructures,which can be varied by controlling synthesis strategy and conditions [10]. In the past decade,a number of synthesis methods,such as co-precipitation [11],sol-gel technique [12],hydrothermal reaction [13],etc. were developed for preparing nanostructured MnO2 with morphologies in a broad range including plates,urchin-like structures,spheres, flowers,cubes,wires,rods,belts,tubes,etc. Depending on how the repeated MnO6octahedron units in MnO2 lattices share their faces and edges,the as-synthesized MnO2 nanostructures can be crystallized in different phases (e.g.,α,β,δ,γ,and λ). Most recently we developed a microwave-assisted hydrothermal method that was capable of synthesizing MnO2 nanostructures with well-controlled morphology and crystallinity in high uniformity and purity [14]. Using microwave energy to drive solution-phase reactions is superior to the conventional heating with respect to prompt start-up,uniform temperature distribution in solutions,efficient energy conversion and delivery,easy control over reaction conditions,and possible scaling up [15,16]. We extend the microwave-assistant synthesis to integrate MnO2 nanostructures with other nanostructures to generate hybrid nanomaterials. Synthesis of composite nanomaterials with multiple functionalities represents a most promising direction because novel properties can be achieved due to the coupling between two different types of components. For instance,hybridization of MnO2 nanostructures with superparamagnetic Fe3O4 nanoparticles results in the composite nanostructures that can be mechanically manipulated on-demand with an external magnetic field. In this letter,we report the synthesis of MnO2 microflowers composed of interconnected nanosheets that encapsulate superparamagnetic Fe3O4@SiO2 core-shell nanoparticles through a microwave-assistant heterogeneous hydrothermal reaction. 2. Experimental
Superparamagnetic Fe3O4@SiO2 core-shell nanoparticles were first synthesized through the following multiple steps. Synthesis of Fe3O4 nanoparticles was realized through a combination of controlled hydrolysis and reduction of Fe(III) at elevated temperatures [17]. In detail,FeCl3salt (Aldrich) was hydrolyzed and partially reduced in basic diethylene glycol (DEG,Fisher Scientific) at 220°C. The basicity was adjusted with addition of appropriate amount of NaOH (Fisher Scientific). Poly(acrylic acid) (PAA, MW = 1800,Aldrich) was added to the reaction solution to serve as surface capping molecules that stabilize the as-grown Fe3O4 nanoparticles. The Fe3O4 nanoparticles were then coated with silica shells through a modified Stöber process. In a typical reaction,to 3 mL of aqueous solution containing ~23 mg Fe3O4 nanoparticles was added 20 mL of ethanol (Fisher Scientific). 1 mL of ammonium hydroxide aqueous solution (28%,Fisher Scientific) was added to the nanoparticle solution to adjust the pH value of the solution for controlled hydrolysis of tetraethyl orthosilicate (TEOS,Aldrich). Injection of 0.1 mL of TEOS to the nanoparticle solution initiated the deposition of silica shells on the surfaces of the Fe3O4nanoparticles. Thickness of the resulting silica shells was tuned by terminating the hydrolysis reaction at different reaction times. Mechanical stirring and room temperature were maintained throughout the reaction. The as-prepared Fe3O4@SiO2 core-shell nanoparticles were then washed with ethanol for two times. The nanoparticles were re-dispersed in 20 mL of deionized (DI) water and stored for characterization and other purposes.
Growth of MnO2 microflowers with encapsulation of the Fe3O4@SiO2 core-shell nanoparticles was carried out through the procedure similar to that for growing pure MnO2 microflowers [14]. In detail,2 mL of the stock solution of the Fe3O4@SiO2 core-shell nanoparticles was mixed with 21 mg of KMnO4 (Aldrich), 0.4 mL of 1.2 mol/L HCl aqueous solution (Aldrich),and 3.6 mL of DI water in a 10-mL microwave reaction tube that was then placed in a CEM Discover® microwave reactor. The solution was then heated to 150°C at the maximum ramping rate under the sealed vessel mode with microwave irradiation. The pressure above the reaction solution reached to 95 psi at temperature of 150°C. By controlling the reaction time,the size of the MnO2 nanosheets in the microflowers was able to tune. Reaction temperature and acidity of the reaction solutions were also changed to serve as control experiments. After reaction,the solid products were collected via either centrifugation or magnetic separation followed by washing with DI water.
The as-synthesized samples were characterized with a JEOL JSM-7500F field emission scanning electron microscope for collecting scanning electron microscopy (SEM) images as well as with a JEOL JEM-2100F microscope operated at a voltage of 200 kV for recording transmission electron microscopy (TEM) images and electron diffraction patterns. Energy dispersive X-ray spectroscopy (EDS) was studied with a Thermo Scientific microanalysis system equipped on the JEOL JSM-7500F field emission scanning electron microscope. A VARIAN CARY-50 spectrophotometer was used to measure the UV-vis-NIR spectra of the reaction solution as well as the concentration of methyl orange. 3. Results and discussion
Superparamagnetic Fe3O4@SiO2 core-shell nanoparticles were synthesized according to the strategy well used in our group [18,19,20,21]. Fig. 1A presents a SEM image of randomly assembled core-shell particles on a Si substrate,showing their uniform size. Fig. 1B shows a typical TEM image of the particles,highlighting their core-shell configuration with dark cores and gray shells corresponding to the different mass densities of Fe3O4and SiO2. Each Fe3O4core (inset,Fig. 1B) is an assembly of many crystalline domains with sizes less than 10 nm. Such bimodal length scale in the Fe3O4 nanoparticles is critical for them to preserve superparamagnetism associated with small crystallites at room temperature [17]. As a result,the Fe3O4@SiO2 core-shell nanoparticles can be easily manipulated with an external magnetic field. The SiO2 shells can not only protect the Fe3O4 cores from being degraded in harsh environment (such as dissolution of Fe3O4in acidic solutions),but also provide surfaces compatible with many reactions and compositions due to the easy modification of SiO2 surface chemistry. The EDS spectrum (Fig. 1C) collected from the area shown in Fig. 1A exhibits strong signal of Si that is attributed to the SiO2 shells and the Si substrate,signal of Fe corresponding to the Fe3O4cores,and signal of O originated from both Fe3O4cores and SiO2 shells. The weak signal of C is probably due to the organic contaminations in the course of sample preparation.

| Download: |
| Fig. 1. Characterization of Fe3O4@SiO2 core-shell nanoparticles with (A) SEM,(B) TEM,and (C) EDS. | |
The strong hydrophilicity and high-density negative charges of SiO2 surfaces makes the SiO2 nanoshells ideal as nucleation sites for growing transition metal oxides,for example,MnO2 nanostructures. We have developed a microwave-assistant hydrothermal approach for the synthesis of MnO2 nanostructures with controlled morphology and crystallinity [14,22]. Similar MnO2 nanostructures can also be synthesized in the presence of Fe3O4@SiO2 core-shell nanoparticles,which provide active nucleation sites for condensation of MnO2. MnO2 nanostructures are formed from the hydrothermal reaction between KMnO4and HCl at elevated temperatures in a microwave reactor. When the precursor chemicals are mixed at room temperature,there is no apparent reaction observed. Once the reaction vessel is sealed, quickly heating the solution to 150°C within 1 min with the microwave reactor triggers a redox reaction:
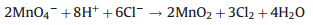
Fig. 2A and B are SEM images of the black powders formed at 5 min,clearly showing flower-like morphology of the MnO2 particles consisting of interconnected nanosheets with thickness of several nanometers. The enlarged image reveals that the spherical Fe3O4@SiO2 core-shell nanoparticles are trapped in the pockets formed among the interconnected nanosheets. The TEM image of an individual MnO2 microflower with many Fe3O4@SiO2 core-shell nanoparticles is presented in Fig. 2C,revealing that most Fe3O4@SiO2 core-shell nanoparticles stay in the center region of the flower. The result indicates that a number of Fe3O4@SiO2 core-shell nanoparticles are slightly aggregated into nanoparticle clusters at the beginning followed by growth of MnO2 nanosheets from the SiO2 surfaces to form microflowers surrounding the Fe3O4@SiO2 core-shell nanoparticles. Because of their thin thickness,the extremely flexible MnO2 nanosheets form conformal contact with the amorphous surfaces of Fe3O4@SiO2 core-shell nanoparticles (Fig. 2D). Selected area electron diffraction (SAED) pattern recorded from a section of a single MnO2 nanosheet highlighted by the circle in Fig. 2D is presented in Fig. 2E,showing two diffraction rings consistent with the (1 1 1) and (0 2 0) reflections of δ-phase MnO2. Analysis of the SAED pattern indicates that the basal surfaces of the MnO2 nanosheets orientate along the [0 0 1] direction and the nanosheets are polycrystalline. The highresolution TEM image of the portion highlighted by the square in Fig. 2D clearly shows the interface between the crystalline MnO2 nanosheet and the amorphous SiO2 nanoshell (Fig. 2F). The crystalline domains in the MnO2 nanosheet are very small with size of only several nanometers,consistent with their polycrystalline property.

| Download: |
| Fig. 2. Characterization of the MnO2 microflowers with encapsulation of Fe3O4@SiO2 core-shell nanoparticles formed at the reaction time of 10 min. (A) SEM image of several microflowers showing that the pockets between the interconnected MnO2 nanosheets are deeper than those shown in Fig. 2A. Most of the Fe3O4@SiO2 core-shell nanoparticles are barely observed due to the deeper pockets. The red arrows highlight some Fe3O4@SiO2 core-shell nanoparticles observed only when the corresponding pockets face the detector. (B) EDS spectrum of the particles shown in (A). (C) TEM image taken at the edge of an individual microflower showing the MnO2 nanosheets are folded (highlighted by the arrows). (D) TEM image of a region with species that are rarely observed in this sample. The inset is the high-resolution TEM image of the area highlighted by the white rectangular box on the nanowire. | |
When the reaction time is elongated,continuous growth of the MnO2 nanosheets can enlarge the size of the flowers. For example, the products formed at 10 min still maintain the flower-like morphology with larger nanosheets (Fig. 3A). The enlarged MnO2 nanosheets form deeper pockets to hide the Fe3O4@SiO2 core-shell nanoparticles,leading them to be barely observed in the SEM images. The EDS spectrum (Fig. 3B) of the hybrid flowers exhibit extra peaks corresponding to Mn and a much stronger peak of O compared to the EDS spectrum shown in Fig. 1C,confirming the hybridization of MnO2 nanosheets and Fe3O4@SiO2 core-shell nanoparticles in the flowers shown in Fig. 3A. Previous studies indicate that overcooking the d-phase MnO2 nanosheets always transforms them into α-phase MnO2 nanostructures with either wire/tube morphology (in solution) [14] or sheet morphology (at dry condition) [23] because the δ-MnO2 nanosheets composed of small crystalline domains represent a metastable phase. We notice that the MnO2 nanosheets start to fold at their edges and merge into thicker stripes as highlighted by arrows in Fig. 3C. The crystalline structure of these stripes is eventually reconstructed into more stable α-phase MnO2 lattices and the stripes continuously grow into α-MnO2 nanowires by consumption of other metastable δ-MnO2 nanosheets. In the product formed at 10 min we can observe the existence of very few nanowires with α-phase lattice (inset,Fig. 3D) as well as fragments of nanosheets. Such fragmentation of nanosheets and phase transformation releases the Fe3O4@SiO2 core-shell nanoparticles from the composite microflowers due to the failure in maintaining the flower-like morphology. Such morphological failure results in the significant decrease in surface areas of MnO2 nanosheets and the loss of ability for magnetic manipulation of MnO2 nanostructures. As a result, reaction for even longer time is not beneficial for maintaining the hybrid mciroflowers of MnO2 nanosheets with embedded Fe3O4@SiO2 core-shell nanoparticles.

| Download: |
| Fig. 3. Characterization of the MnO2 microflowers with encapsulation of Fe3O4@SiO2 core-shell nanoparticles formed at the reaction time of 5 min. (A) Low-magnification SEM image of a number of microflowers randomly assembled on a Si substrate. (B) High-magnification SEM image of microflowers showing that the Fe3O4@SiO2 core-shell nanoparticles sit in the pockets formed between the interconnected MnO2 nanosheets. (C and D) TEM images of an individual microflower with different magnifications. (E) SAED pattern recorded from the area highlighted by the circle in (D). (F) High-resolution TEM image of the small region highlighted by the square in (D). The lattice fringes correspond to the crystalline lattices of δ-phase MnO2. | |
The reduction of KMnO4 strongly depends on the reaction temperature. The reaction becomes dramatically slowly at lower temperature,leading to a difficulty to form MnO2 flowers. Fig. S2 in Supporting information presents the SEM and TEM images of the products formed from reaction at 100°C for 30 min,showing that the Fe3O4@SiO2 core-shell nanoparticles are surrounded/interconnected with amorphous species and nanosheets are barely observed. Variation of acidity of reaction solutions can also lead to morphological change of the MnO2 structures. When HCl is replaced with NaCl,the reaction produces agglomerated MnO2 nanoparticles that serve as a matrix to embed the Fe3O4@SiO2 core-shell nanoparticles (Fig. S3 in Supporting information). The resulting black powders are magnetically responsible,but they cannot be dispersed well in solvents for applications such as catalysis and adsorption that require high surface area. Both temperature and acidity of reaction solutions represent the determining parameters that can influence the reaction kinetics. According to the classic nucleation and growth theory,the difference in reaction kinetics always leads to the formation of nanostructures with different morphologies.
The MnO2 -nanosheet flowers shown in Fig. 3A exhibit strong magnetic response due to the inclusion of the superparamagnetic Fe3O4@SiO2 core-shell nanoparticles. The as-synthesized microflowers in the reaction solution can be easily collected when a magnet is placed near the reactor (Fig. S4 in Supporting information). The black powders can be easily re-dispersed with water after the removal of the magnet. Such magnetic response is beneficial for recovering the MnO2 -nanosheet flowers that are used for applications such as removal of contamination molecules from water. The thin thickness of the MnO2 nanosheets enables the particles shown in Fig. 3A to exhibit very high surface area. Mixing the particles (~15 mg) with 10 mL of 50 mg/L aqueous solution of methyl orange quickly decreases the concentration of methyl orange in the solution,indicating that methyl orange molecules are adsorbed on the MnO2 nanosheets (Fig. 4). The dependences of the absorption spectra (Fig. 4A) and the normalized concentration of methyl orange on the time (Fig. 4B) clearly show that the methyl orange molecules are quickly adsorbed on the MnO2 nanosheets within 5 min. When time is longer than 5 min,the decrease of intensity at the characteristic peak (at 465 nm) of methyl orange becomes much slower while the intensity at the valley increases and eventually a new peak at 340 nm is developed. The change of spectral characteristics indicates that the methyl orange molecules at longer soaking time may be oxidized through a reaction catalyzed by the MnO2 nanosheets [4]. The hybrid Fe3O4@SiO2 -MnO2 microflowers can be easily recovered from the solution with the assistance of a magnet (inset photograph in Fig. 4B). If we can find a way to efficiently remove the adsorbed methyl orange molecules from the MnO2 nanosheets,the recovered hybrid microflowers can be reused.

| Download: |
| Fig. 4. (A) UV-vis absorption spectra of methyl orange after methyl orange was mixed with the synthesized Fe3O4@SiO2 -MnO2 microflowers shown in Fig. 3A for different times (labeled with the legend). (B) The normalized concentration of methyl orange against the original concentration as a function of the soaking time. The insets are photographs of (left) the aqueous solution of methyl orange,(middle) the aqueous solution of methyl orange with hybrid microflowers,and (right) therest solution after adsorption and removal of the microflowers. The hybrid microflowers are dragged to the right side wall of the vial due to the existence of the magnet that can provide a magnet field. | |
In summary,superparamagnetic Fe3O4@SiO2 core-shell nanoparticles can be directly encapsulated in MnO2 microflowers made of interconnected MnO2 nanosheets through a microwave-assistant hydrothermal reduction of KMnO4in acidic aqueous solution in the presence of the Fe3O4@SiO2 core-shell nanoparticles that serve as seeds to prompt nucleation and growth of the MnO2 nanosheets. Successful hybridization of the MnO2 nanosheets and the Fe3O4@SiO2 core-shell nanoparticles enables the synthesized microflowers to exhibit both superparamagnetism corresponding to the Fe3O4@SiO2 core-shell nanoparticles and high surface area associated with the thin MnO2 nanosheets,leading them to be promising in applications such as catalysis,molecule adsorption, etc.
Acknowledgment
Use of the Center for Nanoscale Materials was supported by the U.S. Department of Energy,Office of Science,Office of Basic Energy Sciences,under Contract No. DE-AC02-06CH11357.
| [1] | J. Fei, Y. Cui, X. Yan, et al., Controlled preparation of MnO2 hierarchical hollow nanostructures and their application in water treatment, Adv. Mater. 20 (2008) 452-456. |
| [2] | H. Chen, J. He, Facile synthesis and monodisperse manganese oxide nanostructures and their application in water treatment, J. Phys. Chem. C 112 (2008) 17540-17545. |
| [3] | Y. Zhai, J. Zhai, M. Zhou, S. Dong, Ordered magnetic core-manganese oxide shell nanostructures and their application in water treatment, J. Mater. Chem. 19 (2009) 7030-7035. |
| [4] | A.A. Pandit, R.A. Pawar, D.R. Shinde, Colloidal MnO2 catalysed degradation of two azo dyes methyl red and methyl orange from aqueous medium, Int. J. Sci. Eng. Res. 4 (2013) 1119-1122. |
| [5] | H. Huang, S. Sithambaram, C.H. Chen, et al., Microwave-assisted hydrothermal synthesis of cryptomelane-type octahedral molecular sieves (OMS-2) and their catalytic studies, Chem. Mater. 22 (2010) 3664-3669. |
| [6] | Z. Ai, L. Zhang, F. Kong, et al., Microwave-assisted green synthesis of MnO2 nanoplates with environmental catalytic activity, Mater. Chem. Phys. 111 (2008) 162-167. |
| [7] | X. Lang, A. Hirata, T. Fujita, M. Chen, Nanoporous metal/oxide hybrid electrodes for electrochemical supercapacitors, Nat. Nanotechnol. 6 (2011) 232-236. |
| [8] | M.M. Thackeray, Manganese oxides for lithium batteries, Prog. Solid State Chem. 25 (1997) 1-71. |
| [9] | W. Wei, X. Cui, W. Chen, D.G. Ivey, Manganese oxide-based materials as electrochemical supercapacitor electrodes, Chem. Soc. Rev. 40 (2011) 1697-1721. |
| [10] | S. Devaraj, N. Munichandraiah, Effect of crystallographic structure of MnO2 on its electrochemical capacitance properties, J. Phys. Chem. C 112 (2008) 4406-4417. |
| [11] | T. Brousse, M. Toupin, R. Dugas, et al., Crystalline MnO2 as possible alternatives to amorphous compounds in electrochemical supercapacitors, J. Electrochem. Soc. 153 (2006) A2171-A2180. |
| [12] | X.L. Wang, A.B. Yuan, Y.Q. Wang, Supercapacitive behaviors and their temperature dependence of sol-gel synthesized nanostructured manganese dioxide in lithium hydroxide electrolyte, J. Power Sources 172 (2007) 1007-1011. |
| [13] | G.H. Qiu, H. Huang, S. Dharmarathna, et al., Hydrothermal synthesis of manganese oxide nanomaterials and their catalytic and electrochemical properties, Chem. Mater. 23 (2011) 3892-3901. |
| [14] | T.T. Truong, Y. Liu, Y. Ren, L. Trahey, Y. Sun, Morphological and crystalline evolution of nanostructured MnO2 and its application in lithium-air batteries, ACS Nano 6 (2012) 8067-8077. |
| [15] | D. Dallinger, C.O. Kappe, Microwave-assisted synthesis in water as solvent, Chem. Rev. 107 (2007) 2563-2591. |
| [16] | Y.J. Zhu, F. Chen, Microwave-assisted preparation of inorganic nanostructures in liquid phase, Chem. Rev. 114 (2014) 6462-6555. |
| [17] | J. Ge, Y. Hu, M. Biasini, W.P. Beyermann, Y. Yin, Superparamagnetic magnetite colloidal nanocrystal clusters, Angew. Chem. Int. Ed. 46 (2007) 4342-4345. |
| [18] | Y. Hu, Y. Sun, Stable magnetic hot spots for simultaneous concentration and ultrasensitive SERS detection of solution analytes, J. Phys. Chem. C 116 (2012) 13329-13335. |
| [19] | Y. Hu, Y. Sun, A generic approach for the synthesis of dimer nanoclusters and asymmetric nanoassemblies, J. Am. Chem. Soc. 135 (2013) 2213-2221. |
| [20] | Y. Hu, Z. Li, Y. Sun, Enhanced photocatalysis by hybrid hierarchical assembly of plasmonic nanocrystals with high surface areas, Catal. Today 225 (2014) 177-184. |
| [21] | Y. Hu, Y. Liu, Z. Li, Y. Sun, Highly asymmetric, interfaced dimers made of Au nanoparticles and bimetallic nanoshells: synthesis and photo-enhanced catalysis, Adv. Funct. Mater. 24 (2014) 2828-2836. |
| [22] | Y.G. Sun, L. Wang, Y. Liu, Y. Ren, Birnessite-type MnO2 nanosheets with layered structures under high pressure: elimination of crystalline stacking faults and oriented laminar assembly, Small 10 (2014), http://dx.doi.org/10.1002/ smll.201400892. |
| [23] | Y. Sun, Y. Liu, T.T. Truong, Y. Ren, Thermal transformation of d-MnO2 nanoflowers studied by in-situ TEM, Sci. China Chem. 55 (2012) 2346-2352. |




