The use of transition-metal catalysts for the transfer of carbene units from diazo compounds constitutes a powerful tool in organic synthesis [1]. Although several metals such as rhodium,copper, silver and palladium have been reported to mediate this transformation effectively,how to modify the reactivity and selectivity of the carbene while still maintaining its reactivity toward carbon-hydrogen insertion was the challenge task. Recently,Xi,Shiet al.[2] and Yu,Zhang et al.[3] independently have realized the ligand-controlled gold-catalyzed highly siteselective insertion of a carbene into a carbon-hydrogen bond with diazo compounds. A different chemoselectivity of carbene transfer in C-H functionalization using gold catalyst was also observed for the reaction between phenol and methyl phenyldiazoacetate. Whereas the copper catalyst has been shown to efficiently catalyze the O-H insertion reaction [4] and the dirhodium catalyst exclusively promoted the benzylic C-H insertion [5] (Fig. 1).

| Download: |
| Fig. 1. Highly site-selective carbene transfer of phenols. | |
Xi,Shi and co-workers demonstrated that ‘‘carbophilic carbocations is the key intermediate to give the carbophilic addition product combining gold catalyst with an electron-deficient phosphite ligand. Based on DFT calculations,when using PPh3as the ligand,the bond order of Au-C is 0.490,which the carbene reactivity is revealed. With an electron-deficient phosphite P(OAr)3 as the ligand,the gold-carbene intermediate acts as a ‘‘carbophilic carbocation’’,leading to a highly selective nucleophilic addition on carbon,without addition at typical carbene receptors such as phenol and alkene (Scheme 1).
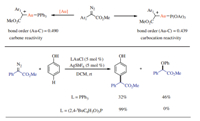
| Download: |
| Scheme 1.Reactivity of gold-carbenoids: carbeneversuscarbocation. | |
The anisole and aniline derivatives are applied to the present highly site-selective C-H functionalization,which is also a challenging issue due to the competitive N-H bond insertion under the metal catalysis. Electron-rich heterocycles,such as benzofuran,pyrrole and indole,are also suitable substrates for this aromatic substitution,whereas the cyclopropanation reaction is dominant when using the dirhodium catalyst is used. This new gold-catalyzed intermolecular highly site-selective direct C-H bond functionalization opens up new exciting opportunities for the functionalization of C-H bonds.
| [1] | M.P. Doyle, R. Duffy, M. Ratnikov, L. Zhou, Catalytic carbene insertion into C-H bonds, Chem. Rev. 110 (2010) 704-724. |
| [2] | Y.M. Xi, Y.J. Su, Z.Y. Yu, et al., Chemoselective carbophilic addition of a-diazoesters through ligand-controlled gold catalysis, Angew. Chem. Int. Ed. 53 (2014) 9817-9821. |
| [3] | Z.Z. Yu, B. Ma, J.M. Chen, et al., Highly site-selective direct C-H bond functionalization of phenols with a-aryl-a-diazoacetates and diazooxindoles via gold catalysis, J. Am. Chem. Soc. 136 (2014) 6904-6907. |
| [4] | X.L. Xie, S.F. Zhu, J.X. Guo, Y. Cai, Q.L. Zhou, Enantioselective palladium-catalyzed insertion a-aryl-adiazoacetates into the O-H bonds of phenols, Angew. Chem. Int. Ed. 53 (2014) 2978. |
| [5] | H.M.L. Davies, Q. Jin, Intermolecular C-H activation at benzylic positions: synthesis of (+)-imperanene and (-)-a-conidendrin, Tetrahedron: Asymmetry 14 (2003) 941-949. |




