b State Key Laboratory of Lake Science and Environment, Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences, Nanjing 210008, China
Organic ligand binding with metals in waters can influence their speciation,which exhibits a substantial effect on the toxicity, mobility and bioavailability [1, 2, 3]. Insight into the ligand-metal interaction is necessary for a better understanding of the environmental behaviors of metals in aquatic environment.
Increasing attention is focused on China Lake Taihu (30°55'40''- 31°32'58'' N and 119°52'32''-120°36'10'' E),a typical eutrophic shallow lake that is suffering serious eutrophication [4, 5]. Large amounts of cyanobacterial blooms occurred regularly each year throughout much of the lake,which caused a high organic matter concentration. To better understand the composition and function heterogeneity,organic matter associated with cyanobacterial blooms should be divided into dissolved organic matter (DOM) and attached organic matter (AOM) based on the binding with algal cells [6]. In addition,increased inflow of untreated or partially treated wastewater also increased the potential metal pollution (such as zinc) [5]. Therefore,comparison of the metal binding behaviors between DOM and AOM is essential for better understanding their environmental fates in the eutrophic algaerich lakes. However,to our best knowledge,no related study has been carried out until now to compare this binding heterogeneity.
Numerous analytical techniques,including ion selective electrode, cathodic stripping voltammetry,one-step resin-exchange, and fluorescence quenching titration had been reported to explore the metal binding properties [3, 7, 8, 9]. Among them,synchronous fluorescence quenching titration was widely utilized due to its rapid,effective and sensitive nature. However,the raw onedimensional spectra usually suffered many overlapped peaks, limiting its application in exploring the subtle binding behaviors. Recent studies have demonstrated that two-dimensional correlation spectroscopy (2D-COS) can be used to resolve peak overlapping problems and more importantly to identify the sequential orders of metal-induced spectral changes [10, 11].
In this study,cyanobacterial blooms were sampled from Lake Taihu,and the commonly found metal zinc in freshwater lake was adopted as the representative metal. The main objectives of this study were therefore (1) to compare the changes in spectral properties of DOM and AOM in response to metal addition and (2) to investigate and reveal the sequential orders of metal-ligand interaction by using quenching titration combined with 2D-COS. Results obtained would enhance our insights into the environmental behaviors of metals in the related aquatic ecosystems. 2. Experimental 2.1. Sample collection and organic matter fractionation
The sample area,called Meiliang Bay,was located in the north region of Lake Taihu in China. Surface cyanobacterial blooms with 0-10 cm water column (0.80 g/L) were sampled by polyethylene bottles,stored on ice,and transported to laboratory within several hours. The samples were filtered through a 0.45 µm PTFE membrane (Xingya Purification Materials Co.,Shanghai,China) for DOM measurement. The residues on the membranes were then carefully scraped,followed by dilution with a 0.05% NaCl solution to the original volume for AOM extraction. Specifically,the cell suspension was firstly heated at 60 8C for 30 min and then centrifuged at 15,000 g for 20 min [6]. The supernatant was filtered through a 0.45 µm PTFE membrane,with the filtered liquid regarded as the AOM fraction. 2.2. Fluorescence titration and complexation modeling
Fluorescence quenching titration was conducted according to Yamashita and Jaffe [2] and Ohno et al. [12]. Experiments were carried out in duplicate by adding 0.1 mol/L Zn2+ to a series of brown sealed vials that contained 50 ml of diluted solution [dissolved organic carbon (DOC) <10 mg/L] using an automatic syringe. It was noted that no more than 50 µL of titrant was added for each vial,making zinc concentrations in the final solutions ranging from 0 to 100 µmol/L in 10 µmol/L steps. The pH was maintained at about 6.0 to avoid precipitation,and all solution samples after titration were shaken at 25 ± 1 8C for 24 h to ensure complexation equilibrium [2].
Fluorescence excitation-emission matrix (EEM) and synchronous fluorescent spectra were measured by a fluorescence spectrometer (Hitachi F-7000,Japan). EEM spectra were gathered with scanning emission (Em) spectra from 250 nm to 550 nm at 2 nm increments by varying the excitation (Ex) wavelength from 200 nm to 450 nmat 10 nm increments. Synchronous spectra were obtained by ranging the Ex wavelengths from 200 nm to 450 nm with a constant offset (60 nm).
Compared with other models,the modified Stern-Volmer equation can partially solve the problems associated with nonlinear fluorescence response by estimating the contribution portion of unquenched fluorescence [1],which was described with the following equation:

The 2D-COS was applied to explore the sequential orders of metal binding with organic ligands. Metal addition was used as the external perturbation,and a set of metal concentration-dependent spectral variations were thus obtained [10].
For the perturbation-induced spectral variation y(x,t) as a
function of a spectral variable (x) and a perturbation variable (t),
the dynamic spectrum  (x,t) is formally defined as follows:
(x,t) is formally defined as follows:
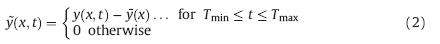

The asynchronous correlation spectroscopy can be obtained
from the dynamic spectrum  (x2,t) and its orthogonal spectrum
(x2,t) and its orthogonal spectrum
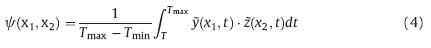
Prior to the analysis of 2D-COS,the fluorescent spectra were firstly normalized by the summed intensities and multiplied by a factor of 1000. The noise components were then removed using the principal component analysis [10, 11, 13]. Afterward,the reconstructed data matrix was progressed using the "2D shige" software (Kwansei-Gakuin University,Japan). 3. Results and discussion 3.1. Spectral characteristics of organic ligands in eutrophic algae-rich lakes
The fluorescence EEM spectra demonstrated that six and two peaks were present inDOM and AOM,respectively (Fig. 1). Peaks A, B,C,D,E,and F were located at Ex/Em of 280/304,220/304,220/440, 270/440,320/370 and 350/440 nm,respectively. It was obvious that fluorescent matters inDOM were composed of protein- (peaks A and B),humic- and fulvic-like substances (peaks C-F),while those in AOM mainly contained protein-like substances. Further analysis showed that,compared with the protein-like peaks in DOM,those in AOM exhibited a slight red-shift along the Em axis. This indicated that the organic components in the two sample types were chemically different [14].
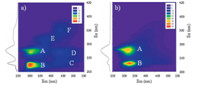
|
Download:
|
| Fig. 1. The typical EEM and synchronous fluorescent spectra for DOM (a) and AOM (b) in the eutrophic algae-rich lakes. | |
As for the synchronous fluorescent spectra,both DOM and AOM were characterized with two peaks at 232 nm and 276 nm; while an additional shoulder peak ranging from 300 nm to 370 nm also occurred in DOM. Peaks at wavelengths between 200 nm to 250 nm,250 nm to 300 nm,and >300 nm were previously ascribed as tyrosine-,tryptophan-,and humic-like substances, respectively [1, 7]. Therefore,it was demonstrated that AOM mainly contained protein-like substances,while both protein-, humic- and fulvic-like substance can be found in DOM. The results observed here were similar to those of the lab-cultured cynobacterium M. aeruginosa [15],indicating the uniform distribution of organic components in respective of the different aquatic environments. 3.2. Variations in one-dimensional fluorescence spectra in response to metal addition
Fig. 2 depicts the variations in fluorescent intensities in response to zinc addition. It was shown that metal addition caused obvious fluorescent quenching both for DOM and AOM, indicating the electronic structural changes in the two fractions due to the formation of ligand-zinc complexes [1, 7]. Metalinduced fluorescence quenching was also reported in the previous studies for sediment DOM [3],soil DOM [7, 13] and leaf litter DOM [1]. Further analysis showed that the behaviors of spectral changes were dependent on sample types as well as the wavelengths investigated. Specifically,the degree of 250-300 nm in DOM was more quenched than that of 200-250 nm; however,as for the AOM,metal addition exhibited a higher quenching effect on 200- 250 nm. The results indicated that tryptophan-like substances in DOM and tyrosine-like substances in AOM were more preferred to bind with zinc than other organic components. In addition,the fluorescent intensities of 250-300 nm in DOM sharply quenched with increasing metal concentration,while that of 200-250 nm tended to decrease slowly in response to metal addition. However, as for the AOM,both the 200-250 and 250-300 nm exhibited a sharp decrease in fluorescence intensities during metal titration. Based on the results,it was suggested that the metal binding behaviors (i.e.,binding affinity,binding sequence) between DOM and AOM were different,which will be discussed in the following section.

|
Download:
|
| Fig. 2. Variations in fluorescent intensities of DOM (a) and AOM (b) in response to zinc addition. The arrows refer to the direction of the increasing zinc concentrations. | |
In order to explore the metal binding heterogeneity of organic ligands,2D-COS was applied in this study (Fig. 3). The synchronous map displayed two autopeaks at 232/232 nm and 276/276 nm along the diagonal line both for DOM and AOM. The synchronous map is a symmetric spectrum with respect to the diagonal line and contains autopeak and/or crosspeak. The autopeak is located at the diagonal line and represents the susceptibility of spectral intensity variation,while the crosspeak locating at the off-diagonal line represents simultaneous or coincidental changes of two different spectral variables [10, 11]. Here both the DOM and AOM were characterized with a positive crosspeak at 276/232 nm. This indicated that spectral changes took place in the same direction at the corresponding wavelength ranges (i.e.,decreasing fluorescent intensity), which was consistent with our previous results (Fig. 2) that fluorescence quenching was observed both for 220-250 nm and 250-300 nm.

|
Download:
|
| Fig. 3. Synchronous and asynchronous 2D fluorescence correlation maps generated from 300 to 200 nm region of the DOM and AOM with zinc addition. (a) Synchronous map for DOM; (b) asynchronous map for DOM; (c): synchronous map for AOM; (d) asynchronous map for AOM. Red represents positive correlations and blue represents negative correlations; higher color intensity indicates a stronger positive or negative correlation. | |
The asynchronous map,on the other hand,reveals the sequential or successive changes of the spectral intensities in response to metal addition. As shown,DOM was characterized with a positive crosspeak at 276/232 nm,while a negative crosspeak locating at 276/232 nm was present in AOM. According to Noda’s rule [10],the sequential orders of fluorescent quenching followed the order: 276 > 232 nm for DOM and 232 > 276 for AOM. The asynchronous maps demonstrated that tryptophan-like substances in DOM exhibited higher binding affinities than tyrosine-like substances,while metal binding with tyrosine-like substances took place prior to that with tryptophan-like substances in AOM. 3.4. Comparison of conditional stability constants with sequential orders derived by 2D-COS
The modified Stern-Volmer model was widely applied to estimate the conditional stability constants (log KM) ofmetal-ligand interaction [3, 7, 13]. In this study,the calculated log KM of tyrosineand tryptophan-like substances for the two sample types ranged from4.51 to 4.83 and 4.52 to 4.93,respectively (Table 1),whichwere comparable with the binding constants derived fromslough [2],soil [7],leaf litter [1] and biofilms [16].
| Table 1 Comparison of the metal binding parameters of organic ligands as calculated using the modified Stern-Volmer equation. |
Further analysis showed that the binding abilities of organic components in the two fractions exhibited obvious differences. The log KM values of peak 276 nminDOMwere clearly higher than that of peak 232 nm (4.93 vs. >4.51),while in the case of AOM, peak 232 nm exhibited a higher stability constants than peak 276 nm (4.83 vs. 4.52). Therefore,the tryptophan-like substances in DOM were characterized with a high metal binding ability, while in AOMtyrosine-like substances exhibited a strong binding affinity. The high metal binding ability of organic ligand in AOM observed in this study definitely manifested its ecological importance for algal cell protection in terms of metal mobility and biotoxicity.
Several interesting points on the sample types and interwavelengths differences in f values could also be observed. Specifically, the f values of 276 nm were clearly higher than those of 232 nm,in respective of the differences in sample types. Meanwhile,the f values of fluorophores in AOM were always higher than that in DOM. This indicated the different binding affinities as well as complexation processes for the organic ligands with different binding strength with algal cells.
At last,it was noted that the log KM values calculated in this study exhibited a decreasing trend in the order of 276 nm > 232 nm for DOM and 232 nm > 276 nm for AOM,which were consistent with the sequential orders determined by the asynchronous correlation spectroscopy. Some previous studies also reported the similar results [7]. Additionally,it was also shown in Table 1 that the log KM value of 232 inDOMwas similar to that of 276 in AOM. However,application of 2D-COS revealed that, although the two fluorophores exhibited similar stability constant, they were characterized with different binding sequences and behaviors. This indicated that the 2D-COS can be used as a powerful tool to obtain more information on metal-ligand interaction as compared with the complexation models. 4. Conclusion
Fluorescent quenching titration combined with 2D-COS was applied to characterize the metal binding properties of organic ligands in the eutrophic algae-rich lakes. Obvious metal-dependent quenching behavior was observed for all organic ligands. Application of 2D-COS revealed the specific metal binding behaviors (i.e.,sequential orders) among various fluorophores. The ranks of conditional stability constants calculated by the modified Stern-Volmer model were consistent with the sequential orders derived by 2D-COS both for the DOM and AOM. Fluorescent quenching titration combined with 2D-COS can also be used to characterize the specific binding behaviors of organic ligands in many other aquatic ecosystems.
AcknowledgmentsThis work was funded by the National Natural Science Foundation of China (Nos. 51479187, 51209192), the China Postdoctoral Science Foundation (Nos. 2014T70505; 2013M 540438), the PAPD, and the State Key Laboratory of Pollution Control and Resource Reuse Foundation (No. PCRRF13011).
| [1] | J. Hur, B.M. Lee, Characterization of binding site heterogeneity for copper within dissolved organic matter fractions using two-dimensional correlation fluorescence spectroscopy, Chemosphere 83 (2011) 1603-1611. |
| [2] | Y. Yamashita, R. Jaffe, Characterizing the interactions between trace metals and dissolved organic matter using excitation-emission matrix and parallel factor analysis, Environ. Sci. Technol. 42 (2008) 7374-7379. |
| [3] | H.C. Xu, G.H. Yu, L.Y. Yang, H.L. Jiang, Combination of two-dimensional correlation spectroscopy and parallel factor analysis to characterize the binding of heavy metals with DOM in lake sediments, J. Hazard. Mater. 263 (2013) 412-421. |
| [4] | H.W. Paerl, H. Xu, M.J. McCarthy, et al., Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): the need for a dual nutrient (N & P) management strategy, Water Res. 45 (2011) 1973-1983. |
| [5] | J. Shen, E.F. Liu, Y.X. Zhu, S.Y. Hu, W.C. Qu, Distribution and chemical fractionation of heavy metals in recent sediments from Lake Taihu, China, Hydrobiologia 581 (2007) 141-150. |
| [6] | H.C. Xu, H.L. Jiang, UV-induced photochemical heterogeneity of dissolved and attached organic matter associated with cyanobacterial blooms in a eutrophic freshwater lake, Water Res. 47 (2013) 6506-6515. |
| [7] | J. Hur, B.M. Lee, Comparing the heterogeneity of copper-binding characteristics for two different-size soil humic acid fractions using fluorescence quenching combined with 2D-COS, Scientific World J. 11 (2011) 1865-1876. |
| [8] | H.C. Xu, Z.S. Yan, H.Y. Cai, G.H. Yu, H.L. Jiang, Heterogeneity in metal binding by individual fluorescent components in a eutrophic algae-rich lake, Ecotoxicol. Environ. Saf. 98 (2013) 266-272. |
| [9] | R.A. Saar, J.H. Weber, Comparison of spectrofluorometry and ion-selective electrode potentiometry for determination of complexes between fulvic acid and heavy-metal ions, Anal. Chem. 52 (1980) 2095-2100. |
| [10] | I. Noda, Y. Ozaki, Two-Dimensional Correlation Spectroscopy: Applications in Vibrational and Optical Spectroscopy, John Wiley and Sons Inc., London, 2004. |
| [11] | G.H. Yu, Z. Tang, Y.C. Xu, Q.R. Shen, Multiple fluorescence labeling and two dimensional FTIR-13C NMR heterospectral correlation spectroscopy to characterize extracellular polymeric substances in biofilms produced during composting, Environ. Sci. Technol. 45 (2011) 9224-9231. |
| [12] | T. Ohno, A. Amirbahman, R. Bro, Parallel factor analysis of excitation-emission matrix fluorescence spectra of water soluble soil organic matter as basis for the determination of conditional metal binding parameters, Environ. Sci. Technol. 42 (2008) 186-192. |
| [13] | G.H. Yu, M.J. Wu, G.R. Wei, et al., Binding of organic ligands with Al(III) in dissolved organic matter from soil: implications for soil organic carbon storage, Environ. Sci. Technol. 46 (2012) 6102-6109. |
| [14] | G.P. Sheng, H.Q. Yu, Characterization of extracellular polymeric substances of aerobic and anaerobic sludge using three-dimensional excitation and emission matrix fluorescence spectroscopy, Water Res. 40 (2006) 1233-12239. |
| [15] | H.C. Xu, H.Y. Cai, G.H. Yu, H.L. Jiang, Insights into extracellular polymeric substances of cyanobacterium Microcystis aeruginosa using fractionation procedure and parallel factor analysis, Water Res. 47 (2013) 2005-2014. |
| [16] | D.Y. Zhang, X.L. Pang, K.M.G. Mostofaa, et al., Complexation between Hg(II) and biofilm extracellular polymeric substances: an application of fluorescence spectroscopy, J. Hazard. Mater. 175 (2010) 359-365. |





