b College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China;
c Institute of Process Engineering and Chinese Academy of Sciences, Beijing 100190, China;
d College of Chemistry and Materials, Hebei Normal University, Shijiazhuang 050016, China;
e Two Five Eight Health Technology Co., Ltd, Beijing 100045, China;
f The Third School of Clinical Medicine of Peking University, Beijing 100083, China;
g Ninghai Doubly Advanced Material Co., Ltd, Ninghai 315602, China;
h Department of Materials Science and Engineering, University of Delaware, Newark, DE 19716, USA
Metal ions exhibit remarkable ability to coordinate with organic ligands. The coordination is extensively utilized in the construction of supra-molecular system [1, 2] and fabrication of organic-inorganic hybrid materials [3, 4, 5, 6, 7, 8]. Moreover,the coordination between metal ions and organic ligands is helpful in shedding light on the physico-chemical nature of molecular catalyst. In our previous work [9, 10, 11, 12, 13],a systematic investigation has been conducted on the coordination between metal ions and amide group of polyamides. Upon the coordination,metal ions exhibit considerable electron-withdrawing effect on the oxygen atom of carbonyl group. As a result,the bond strength of the carbonyl groups decreases,leading to the lower wavenumber shift of amide I band. Based on the above result,we have developed a new technique of manufacturing fine denier polyamide 6 fiber by using lanthanide complexes as additive during the melt spinning process [14, 15].
However,it is not clear whether coordination occur between metal ions and other carbonyl compounds such ketone,ester,etc. In conventional 1D UV-vis spectra,metal ions cannot produce observable band-shift of the carbonyl band of organic compounds. One possible reason for this phenomenon is that band-shift caused by coordination is too subtle to be observed in convention 1D spectra. Thus,advanced spectroscopic technique with enhance spectral resolution is need to shed light on interaction between metal ions and carbonyl groups.
Two-dimensional correlation spectroscopy is a suitable spectroscopic technique to probe interaction between metal ions and carbonyl group. Generally,the cross peaks of 2D correlated spectra can be used to characterize intermolecular interactions [16]. However,interfering cross peaks due to other sources of correlation,such as concentration variations of solutes,may also arise even if there is no intermolecular interaction. This problem makes it difficult to directly use the cross peaks in conventional 2D spectrum as a reliable criterion to judge whether intermolecular interaction actually occurs or not.
To address the above problem,we have developed orthogonal sample design scheme (OSD) [17, 18, 19, 20, 21],asynchronous orthogonal sample design scheme (AOSD) [22, 23, 24],double orthogonal sample design scheme (DOSD) [25] and double asynchronous orthogonal sample design scheme (DAOSD) [26, 27, 28, 29, 30] to remove the interfering parts completely. As a result,portion of spectral deviations from the Beer-Lambert’s law,which are relevant to intermolecular interactions among different solutes can be clearly manifested as specific cross peaks in the corresponding two-dimensional correlation spectrum. We have applied the OSD and DAOSD approaches to investigate interaction between metal ions and organic compounds containing ketone or ester groups [20, 27, 28]. Experimental results demonstrated that the variation on the d-d or f-f transition peaks of metal ions in UV-vis spectra and subtle spectral variation on the functional groups of organic ligands in FTIR spectra can be visualized via a series of cross peaks in the 2D correlation spectra.
However,many metal ions such as calcium,lanthanum, aluminum,etc.,have closed electronic shells and these ions are dump ion since neither d-d nor f-f transition peak can be observed in convention UV-vis spectra. Thus,the OSD,DOSD,AOSD and DAOSD approaches are not suitable to study interaction of these dump ions and organic compounds with carbonyl groups.
Recently,we have proposed a new approach to investigate intermolecular interaction between two solutes dissolved in the same solution,in which only one solute possesses observable characteristic peak [31]. In this paper,we apply the new approach to study the interaction between carbonyl group from butanone and metal ions such as Ca2+ and Al3+.
2. ExperimentalMaterials: Calcium chloride,aluminum chloride and butanone were of A.R. grade and purchased from Beijing Chemical Company. Acetonitrile was of HPLC grade and purchased from Dikma Technologies Inc.
Instrument: The UV-vis spectra were recorded on a Lambda35 UV-vis spectrophotometer (Perkin Elmer) and the spectra were measured at a scanning rate of 60 nm/min.
Programming: The 2D asynchronous spectra were calculated by using the UV-vis 1D spectra based on the algorithm by Noda [32] using the software of MATLAB (The Math Works Inc.).
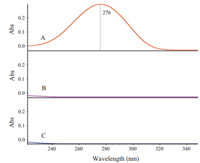
3. Results and discussionFig. 1 shows a typical UV-vis spectrum of butanone (trace A) dissolved in acetonitrile. A peak around 276 nm that is assigned to n-π* transition band is utilized as a probe to reflect coordination between carbonyl group of butanone and metal ions. To study the coordination between butanone and metal salts,suitable solvent should be adopted. Firstly,we tried to use acetonitrile as a solvent to dissolved butanone since acetonitrile has very weak end absorption in the UV-vis spectrum (For comparison,a UV-vis spectrum of acetonitrile (trace B) is also shown Fig. 1). This provides an ideal background for constructing 2D asynchronous spectrum. However,the solubility of calcium chloride in acetonitrile is very low. As a matter of fact,we have mixed saturated acetonitrile solution of CaCl2 with aqueous solution of Na2CO3 (the concentration of Na2CO3 is 1.0 mol/L),no white precipitate of CaCO3 was observed. To solve the problem,we adopted wateracetonitrile mixture as a solvent. The solubility of both butanone and metal salts are enough for constructing 2D asynchronous spectrum. A UV-vis spectrum of water (trace C) shown Fig. 1 indicates that the interference from the absorption of water on the 2D asynchronous spectrum can also be avoided. Therefore,the chemical system studied here is a series of solutions. The solvent is acetonitrile/water mixture while butanone and metal salts are solutes. Although the contribution from the solvent to the characteristic peaks of the solutes is absent,interaction between solvent and solutes still occurs. Especially,hydrogen bond may occur between water and the carbonyl of butanone. As a matter of fact,the n-π* band of carbonyl group of butanone undergoes observable band-shift when the content of water is high (data not shown). Thus,a challenge of this work is to differentiate spectral change on the carbonyl band caused by hydrogen bond from that induced by coordination to metal ions.
|
Download:
|
| Fig. 1. UV spectra of butanone (trace A) dissolved in acetonitrile,acetonitrile (trace B) and water (trace C). | |
The basic idea to address the above problem is as follows: when butanone or metal salts is dissolved in the mixed solvent alone,the solute and its solvating layer can be regarded as a separate entity. Under a suitable concentration range of the solutes,the integrity of the separate entity remains virtually undisturbed and thus the separate entity also follows the Beer-Lambert Law. When two solutes co-exist in the same solution,the integrity of the separate entity is destroyed via interaction between the two solutes. Consequently,spectral signal that deviates from the Beer-Lambert Law produces cross peaks in 2D asynchronous spectrum. In experiment,we adopt water/acetonitrile mixture (the weight ratio between water and acetonitrile is fixed at 2.954) as the solvent. The reason for this selection is the contents of water and acetonitrile are much larger than the contents of solute. As a result,there are enough water and acetonitrile molecules to form a solvating layer with invariant chemical composition. Under this condition,a series of solutions containing different amount of butanone were prepared and UV-vis spectra were recorded. As we expected, the peak position of the n-π* transition band of butanone is fixed 269 nm (Fig. 2a). That is to say,the hydrogen bond on butanone is saturated by water in so that the corresponding n-π* band does change when the concentration of butanone undergoes variation. Moreover,a good linearity (R2 > 0.999) can be observed between absorbance of the n-π* transition band and concentration (Fig. 2b) when the suitable concentration of butanone is between 0 and 5.2 × 10-2 mol/L. Based on the above result,2D asynchronous spectra of butanone/metal salts system are generated from a series of solution whose concentration of butanone is between 0 and 5.2 × 10-2 mol/L.

|
Download:
|
| Fig. 2. (a) UV spectrum of butanone dissolved in acetonitrile/water mixture solution. (b) Fitting results of linear relationship between absorbance and concentration of butanone (R2 = 0.999). | |
Then we use 2D asynchronous spectrum to investigate coordination between metal ions and butanone. First,2D asynchronous spectrum generated from a series of solution containing butanone only. Four acetonitrile/water mixture solutions were prepared and the concentrations of butanone are listed in Table 1. UV-vis spectra of solutions are shown in Fig. 3a and the corresponding 2D asynchronous correlation spectrum is shown in Fig. 3b. The 2D asynchronous spectrum is dominated by noise and no apparent cross peaks can be observed. That is to say,the experimental setup in the present work effectively suppresses cross peaks caused by hydrogen bonds between carbonyl group and water,thereby provide an idea background to study coordination between metal ions and butanone by using 2D asynchronous spectra.
| Table 1 The concentrations of butanone in the acetonitrile/water solutions. |

|
Download:
|
| Fig. 3. (a) UV spectra of the four butanone solutions,acetonitrile/water mixture are used as a solvent. The concentrations of butanone are shown in Table 1. (b) 2D asynchronous spectrum of butanone dissolved in acetonitrile/water mixture. | |
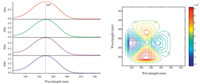
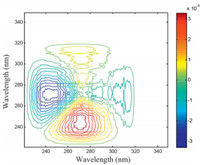
Subsequently,CaCl2 was introduced into four acetonitrile/ water solutions of butanone. The concentration of calcium chloride and butanone of the solutions are listed in Table 2. UV-vis spectra of the solutions were given in Fig. 4a. The presence of calcium ion only produces a subtle variation on the n-π* band,which is difficult to be observed. Cross peaks can be observed in the corresponding 2D asynchronous spectrum shown in Fig. 4b,demonstrating that coordination between Ca2+ and carbonyl group of butanone occur in the solution. Moreover,the pattern of cross peaks in Fig. 4b is significantly different from that in Fig. 3b. This results indicates that the variation of on the n-π* transition band caused by coordination can be differentiated from that from hydrogen bond in the 2D asynchronous spectrum. In Fig. 4b,for cross peaks form a butterfly pattern. This result suggest that coordination bring about a red-shift on the n-π* band. In addition,the bandwidth the n-π* band decreases upon coordination. Similar phenomenon was also observed in butanone/aluminum chloride system (Fig. 5). Although the content of water is overwhelming larger than that of metal ions,cross peaks that are relevant to coordination can be observed. The result demonstrates that carbonyl group of butanone exhibit remarkable tendency to coordination with metal ions in aqueous environment.
| Table 2 The concentrations of butanone and calcium chloride of the solutions. |
|
Download:
|
| Fig. 4. (a) UV spectra of four acetonitrile/water solutions containing butanone/CaCl2 for constructing 2D asynchronous spectrum. The concentrations of butanone and CaCl2 are shown in Table 2. (b) 2D asynchronous spectrum of butanone/CaCl2 system dissolved in acetonitrile/water mixture. | |
|
Download:
|
| Fig. 5. 2D asynchronous spectrum of butanone/AlCl3 system dissolved in acetonitrile/water mixture. | |
We use 2D asynchronous spectrum to characterize coordination between carbonyl group of butanone and metal ions in water/ acetonitrile solution. The n-π* band of butanone is utilized to reflect coordination behavior based on an approach proposed in our recent paper even if the metal ions do not possess any characteristic peak. Experimental results demonstrate that Ca2+, Al3+ show considerable ability to coordinate with the carbonyl group of butanone dissolved in water/acetonitrile mixture.
AcknowledgmentThis project is financially supported by the National Natural Science Foundation of China (No. 51373003) and Beijing Natural Science Foundation (No. 2122059).
| [1] | Y.F. Liu, J.C. Su, W.H. Li, J.G. Wu, First hydrotalcite-like sulfonate coordination network incorporating robust cationic layers and flexible interlayer interactions, Inorg. Chem. 44 (2005) 3890-3895. |
| [2] | X.H. Hua, Q.H. Pan, L. Yu, et al., Preparation and spectroscopic characterization of two HoCl3-galactitol complexes and one ErCl3-galactitol complex, J. Mol. Struct. 998 (2011) 225-232. |
| [3] | J. Liu, S.X. Liu, Y.L. Gao, et al., On the interaction between PVP and europium benzenesulfonate, Spectrosc. Spectr. Anal. 33 (2013) 1487-1490. |
| [4] | S.X. Liu, C.F. Zhang, Y.H. Liu, et al., Coordination between yttrium ions and amide groups of polyamide 6 and the crystalline behavior of polyamide 6/yttrium composites, J. Mol. Struct. 1021 (2012) 63-69. |
| [5] | S.X. Liu, C.F. Zhang, E. Proniewicz, et al., Crystalline transition and morphology variation of polyamide 6/CaCl2 composite during the decomplexation process, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 115 (2013) 783-788. |
| [6] | S.C. Xu, B.C. Hu, W.Y. Zhou, et al., The synthesis, self-assembly and electrocatalytic property of a novel disulphide derivatised cobalt(II) deuteroporphyrin, Chin. Chem. Lett. 23 (2012) 157-160. |
| [7] | Y.H. Hui, C.M. Chen, Z.F. Xie, Catalytic conjugate addition of indole to a,bunsaturated ketones by Co(ClO4)2 6H2O/bis-Schiff base complexes, Chin. Chem. Lett. 23 (2012) 525-528. |
| [8] | E.T. Hennessy, T.A. Betley, Complex N-heterocycle synthesis via iron-catalyzed, direct C-H bond amination, Science 340 (2013) 591-595. |
| [9] | Y.Z. Xu, W.X. Sun, W.H. Li, et al., Investigation on the interaction between polyamide and lithium salts, J. Appl. Polym. Sci. 77 (2000) 2685-2690. |
| [10] | W.X. Sun, X.B. Hu, Y.Z. Xu, et al., Study on the interaction between polyamide and lanthanide ions, Acta Chim. Sin. 58 (2000) 1602-1607. |
| [11] | Y.Z. Xu, J.G. Wu, W.X. Sun, et al., A new mechanism of Raman enhancement and its application, Chem. Eur. J. 8 (2002) 5323-5331. |
| [12] | A.F. Xie, D.L. Tao, Z.B. Zhang, et al., The coordination and phase separation in nylon-copper chloride system, J. Mol. Struct. 613 (2002) 67-71. |
| [13] | Y.J. Wu, Y.Z. Xu, D.J. Wang, et al., FT-IR spectroscopic investigation on the interaction between nylon 66 and lithium salts, J. Appl. Polym. Sci. 91 (2004) 2869-2875. |
| [14] | C.F. Zhang, G.Q. Lai, Y.F. Liu, et al., China Patent, ZL 200710099455.3, 2010. |
| [15] | C.F. Zhang, Y.H. Liu, S.X. Liu, et al., Crystalline behaviors and phase transition during the manufacture of fine denier PA6 fibers, Sci. China, Ser. B: Chem. 52 (2009) 1835-1842. |
| [16] | I. Noda, Generalized two-dimensional correlation method applicable to infrared, Raman, and other types of spectroscopy, Appl. Spectrosc. 47 (1993) 1329-1336. |
| [17] | J. Qi, H.Z. Li, K. Huang, et al., Orthogonal sample design scheme for two-dimensional synchronous spectroscopy and its application in probing intermolecular interactions, Appl. Spectrosc. 61 (2007) 1359-1365. |
| [18] | Y.H. Liu, C.F. Zhang, S.X. Liu, et al., Modified orthogonal sample design scheme to probe intermolecular interactions, J. Mol. Struct. 883 (2008) 124-128. |
| [19] | J. Chen, C.F. Zhang, H.Z. Li, et al., Patterns of cross peaks in 2D synchronous spectrum generated by using orthogonal sample design scheme, J. Mol. Struct. 883 (2008) 129-136. |
| [20] | J. Qi, K. Huang, X.X. Gao, et al., Orthogonal sample design scheme for twodimensional synchronous spectroscopy: application in probing lanthanide ions interactions with organic ligands in solution mixtures, J. Mol. Struct. 883 (2008) 116-123. |
| [21] | H.Z. Li, D.L. Tao, J. Qi, et al., Dipole-dipole interactions in solution mixtures probed by two-dimensional synchronous spectroscopy based on orthogonal sample design scheme, Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 124 (2014) 697-702. |
| [22] | X.P. Li, Q.H. Pan, J. Chen, et al., Asynchronous orthogonal sample design scheme for two-dimensional correlation spectroscopy (2D-COS) and its application in probing intermolecular interactions from overlapping infrared (IR) bands, Appl. Spectrosc. 65 (2011) 901-917. |
| [23] | X.P. Li, Q. Bi, S.X. Liu, et al., Improvement of the sensitivity of the two-dimensional asynchronous spectroscopy based on the AOSD approach by using a modified reference spectrum, J. Mol. Struct. 1034 (2013) 101-111. |
| [24] | X.P. Li, S.X. Liu, J. Chen, et al., The influence of changing the sequence of concentration series on the 2D asynchronous spectroscopy generated by the asynchronous orthogonal sample design (AOSD) approach, Vib. Spectrosc. 60 (2012) 212-216. |
| [25] | C.F. Zhang, K. Huang, H.Z. Li, et al., Double orthogonal sample design scheme and corresponding basic patterns in two-dimensional correlation spectra for probing subtle spectral variations caused by intermolecular interactions, J. Phys. Chem. A 113 (2009) 12142-12156. |
| [26] | J. Chen, Q. Bi, S.X. Liu, et al., Double asynchronous orthogonal sample design scheme for probing intermolecular interactions, J. Phys. Chem. A 116 (2012) 10904-10916. |
| [27] | Y.L. Gao, J. Liu, Y.H. Liu, et al., Characterization of the coordination between Nd3+ and ester groups by using double asynchronous orthogonal sample design approach, J. Mol. Struct. 1069 (2014) 205-210. |
| [28] | J. Liu, Y.L. Gao, L.R. Zheng, et al., Coordination between cobalt (II) ion and carbonyl group in acetone probed by using DAOSD approach, J. Mol. Struct. 1069 (2014) 217-222. |
| [29] | Q. Bi, J. Chen, X.P. Li, et al., Investigation on the dipole-dipole interactions between tetramethylurea and acetonitrile by two-dimensional asynchronous spectroscopy, J. Mol. Struct. 1069 (2014) 264-271. |
| [30] | Q. Bi, J. Chen, X.P. Li, et al., A method based on the DAOSD approach to estimate the variation of the peak position and bandwidth caused by intermolecular interactions, J. Mol. Struct. 1069 (2014) 211-216. |
| [31] | X.P. Li, X.K. Fan, K. Huang, et al., Characterization of intermolecular interaction between two substances when one substance does not possess any characteristic peak, J. Mol. Struct. 1069 (2014) 127-132. |
| [32] | I. Noda, Determination of two-dimensional correlation spectra using the Hilbert transform, Appl. Spectrosc. 54 (2000) 994-999. |






