b Pohang Accelerator Laboratory, Pohang 790-784, Republic of Korea;
c Department of Chemistry, Kangwon National University, Chunchon 200-701, Republic of Korea
Surface grafted polymer films on various substrates have attracted considerable research attention because of their unique surface properties,such as friction,biocompatibility,wettability, and corrosion resistance [1, 2, 3, 4, 5, 6]. Surface grafted polymer films can be prepared by attaching one chain end group of polymers to a variety of substrates through a covalent bond [7, 8, 9]. To obtain surface-grafted polymer films,in situ polymerization processes are conducted on the initial modified surface of substrates. A range of polymerization methods,such as atom transfer radical polymerization (ATRP) [6, 7, 8, 9, 10],reversible addition fragmentation chain transfer (RAFT) polymerization [11, 12] and ring opening metathesis polymerization (ROMP) [13, 14],using vinyl derivatives as the monomers have been studied to obtain versatile,reliable and controllable vinyl polymer film surfaces. The ring opening polymerization (ROP) of N-carboxy anhydride (NCA) derivatives as monomers to prepare surface-grafted polypeptides has been examined [15, 16, 17, 18].
Among the surface grafted polymers,polypeptides-grafted films have attracted attention because of their potential applications, such as biomedical,anti-fouling and stimuli responsive materials [15, 19, 20, 21]. The ordered secondary structures α-helix and β-sheet) and random coil conformation provide extraordinary thin film properties. In addition,the conformational transition can be obtained easily changing the external stimuli,such as pH, temperature and solvent [16, 17, 18, 22]. Many research groups have reported the basic properties of surface-grafted polypeptide films and applications,such as chiral separating membranes,optical switches and biosensors. Despite the extraordinary features of surface grafted polypeptide films,the structural changes in grafted peptide films by external stimulation are not completely understood. Therefore,it is important to reveal the chemical behaviors of the secondary structure and random coil conformational transition upon changes in external stimulation for surface grafted polypeptide films.
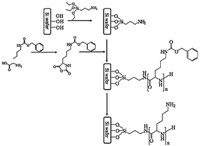
In this study,a grafted film composed of poly(L-lysine) (PLL), which has a peptide main chain and amino side chains,was fabricated using a ring opening surface-initiated polymerization method with a NCA of N-carbobenzyloxyl-L-lysine (CBL) (Fig. 1) [18]. This study examined the pH-induced structural changes in surface immobilized PLL by Fourier transform infrared (FTIR) spectroscopy. FTIR spectroscopy is a sensitive technique that can be used to identify the spectral changes in the proteins including the amide group in the main chain and the alkyl and amino groups in the side chain of PLL [23, 24, 25]. Moreover,2D correlation spectroscopy was conducted to closely investigate the pH-induced structural changes in the surface immobilized PLL film. 2D correlation spectroscopy can be used to analyze the pH-induced spectral changes in the amide group in the main chain and the alkyl and amino groups in the side chain as well as to enhance the spectral resolution in the N-H stretching vibrations related to the amide group in the main chain and the amino group in the side chain [26, 27, 28]. Therefore,2D correlation analysis of the PLL FTIR spectra can be used to examine closely the pH-induced spectral changes in the characteristic bands of the peptide unit in the main chain and the alkyl and amino groups in the side chain of the surface immobilized PLL film after different pH treatments.
|
Download:
|
| Fig. 1. Synthetic scheme of surface immobilized poly(L-lysine) (PLL). | |
Materials and preparation of substrate: N6carbobenzyloxy- L-lysine (CBL,Aldrich),triphosgene (Aldrich),n-hexane (Samchun), toluene (Aldrich),and 3-(aminopropyl)triethoxysilane (APS, Aldrich) were used as received. The solvents for synthesis,i.e., dimethylformamide (DMF) and ethyl acetate (EA),were purchased from Aldrich,purified by distillation over calcium hydride and stored over 4Å molecular sieves. All buffer solutions were purchased from Duksan Chemicals. An oxidized silicon (Si) wafer was cut into 2 cm × 2 cm. After sonication with ethanol for 10 min and rinsing with de-ionized (DI) water,the Si substrates were immersed in a ‘‘piranha solution’’ (H2SO4/30% H2O2 = 7/3 (v/v)), (Caution: Piranha solutions are extremely dangerous and should be used with extreme caution) at 80 8C for 10 min. The cleaned substrates were rinsed with DI water and dried with N2. Silanization of the Si wafer was conducted by immersing the Si substrates into a 2 wt% solution of an APS solution in toluene. The silanized substrates were rinsed with ethanol and distilled water and dried with N2.
Synthesis of N6carbobenzyloxy-L-lysine N-carboxylic anhydride: N6Carbobenzyloxy-L-lysine N-carboxylic anhydride (CBLNCA) was synthesized from a reaction of CBL and triphosgene. A mixture of CBL and triphosgene in EA was heated under reflux for 12 h under a N2 atmosphere. The resulting pale yellow solution was cooled to room temperature,and washed with cold deionized water. The organic layer was dried with MgSO4 and concentrated. The crude product was recrystallized from n-hexane to obtain white crystals. The product was identified by proton nuclear magnetic resonance (1H NMR) spectroscopy (model AM300, Bruker) from a solution in chloroform-d1 (CDCl3). 1H NMR (300 MHz,CDCl3): δ 7.1-7.3 (m,5H,Ph-H),6.8 (s,1H,NH-),5.0 (s,2H,CH2-benzylic),4.8 (t,1H,O-C-H),4.1 (t,1H,C-H),3.1 (d,2H, CH2-NH),1.9 (m,1H,β-CH),1.6 (m,2H,-RCH2),1.3 (m,4H, -CH2CH2).
Preparation of surface immobilized poly(L-lysine): The surface immobilization of poly(N6carbobenzyloxy-L-lysine) (PCBL) was conducted using CBLNCA and APS silanized Si wafer in anhydrous DMF. The APS-modified Si wafer was immersed in a 100 mmol/L solution of CBL at ambient temperature under a N2 atmosphere for 12 h. After polymerization,the substrates were rinsed with copious amounts of DMF. The substrates were sonicated several times in DMF to completely remove the physisorbed polymer and unreacted CBL from the surface,rinsed with ethanol,and then dried with N2. Surface immobilized poly(L-lysin) (PLL) was prepared by a deprotecting reaction of N6carbobenzyloxy groups from immobilized PCBL on a Si wafer. The PCBL-immobilized Si wafer was immersed in a HBr/benzene solution for 2 h [18]. After the reaction,the substrates were rinsed with acetone and distilled water,and dried with N2.
2.2. MeasurementsThe 1H NMR (Bruker AM 300) spectrum was obtained at room temperature. FTIR spectroscopy was conducted on a Bruker Vertex 80/v FTIR spectrometer equipped with an ATR accessory (PIKE) at the Pohang Accelerator Laboratory (PAL). The FTIR spectra were recorded at a 4 cm-1 resolution using a liquid-nitrogen-cooled mercury cadmium telluride (MCT) detector. The 2D correlation spectra were obtained using an algorithm based on the numerical method reported by Noda. 2D correlation analysis was performed after a baseline correction. A subroutine called KG2D,which was written in Array Basic language (GRAMS/386; Galactic Inc.,NH), was used in 2D correlation analysis [29].
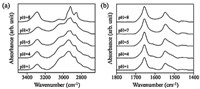
3. Results and discussionFig. 2 shows the pH-dependent FTIR spectra of the surface immobilized PLL in the region of 3500-2650 cm-1 and 1800- 1400 cm-1. The intensities of the band at 3030 cm-1 decreased with increasing pH (Fig. 2(a)). In particular,the intensities of the band at 3030 cm-1 decreased abruptly between pH 7 and 8. In contrast,the intensities of the band at 3270 cm-1 increased with increasing pH from 1 to 8. The bands at 3030 and 3270 cm-1 were assigned to the N-H stretching vibration of the protonated amino group (NH3+) and the deprotonated amino group (NH2) in the side chain,respectively [26, 30]. Therefore,these spectral changes suggest an alteration of the protonation state of the amino group in the PLL side chain depending on the pH. The amino group in the PLL side chain was protonated at acidic and neutral pH. The amino group in the PLL side chain was deprotonated at basic pH,as described in previously [18, 26, 30, 31].
|
Download:
|
| Fig. 2. FTIR spectra of the surface immobilized poly(L-lysine) in the region of (a) 3500-2650 cm-1 and (b) 1800-1400 cm-1 at various pH. | |
In addition,the bands due to the asymmetric and symmetric C-H stretching vibrations of the CH2 group in the PLL side chain showed spectral changes with increasing pH. The asymmetric and symmetric C-H stretching vibrations of CH2 groups were observed at 2940 and 2870 cm-1 at acidic pH. On the other hand,the stretching vibration of the CH2 group were detected at 2922 and 2854 cm-1 at basic pH (pH 8). The band positions of the CH2 stretching vibration are conformation sensitive,where lower wavenumbers indicate ordered conformations [32]. This suggests that the alkyl chain in the surface immobilized PLL side chain at basic pH exhibits a more ordered conformation than at acidic and neutral pH. The spectral changes in the alkyl group occurred abruptly between pH 7 and 8,as shown in the spectral changes in the amino group in the side chain.
In Fig. 2(b),the characteristic bands assigned to the stretching vibration of the C55O group (amide I) and the in-plane bending vibration of the NH group (amide II) in the peptide unit of the PLL main chain were detected at 1654 and 1547 cm-1,respectively [18, 24, 25, 33]. The amide I band is quite sensitive to the conformational transition of the peptide linkage. The β-sheet structure is located at 1610-1640 cm-1 and 1680-1690 cm-1 [33]. The random coil and a-helix structure were observed at 1650- 1660 cm-1 and 1640-1650 cm-1,respectively [18, 33]. The conformational transition of the secondary structure of PLL among the a-helix,β-sheet and random coil were induced by the pH [18, 31, 33]. At low pH,PLL consists of the random coil structure and PLL can exist as the a-helix and/or β-sheet at high pH depending on the external environment [18, 33]. Therefore,the band at 1654 cm-1 can be assigned to the random coil structure at acidic pH [18]. On the other hand,the random coil and a-helix structure could not be resolved in the present study due to overlap of the band caused by the random coil with a-helix structure. In addition,the bands due to the β-sheet structure could not be observed between pH 1 and 8.
2D correlation spectroscopic analysis of the FTIR spectra was applied to further understand the pH-induced spectral changes in the surface immobilized PLL films in the range. The 2D correlation spectra were constructed from the FTIR spectra measured at pH 1-8. 2D correlation analysis results in the amide I and II region did not provide new insights. Here,we only focused on the 2D correlation spectra in the region of 3500-2650 cm-1. Fig. 3 shows the synchronous and asynchronous 2D IR correlation spectra measured over the range,3500-2650 cm-1. According to the synchronous 2D correlation spectrum (Fig. 3(a)),the change in intensity at 3030 cm-1 suggests that the protonation state of the amino group in the side chain is strongly affected by changes in pH. Moreover,the change in the intensity at 2870 cm-1 showed that the alkyl group in the side chain is also strongly affected by the pH. The decrease in protonation of the surface-immobilized PLL film with increasing pHinduced spectral changes in the amino group as well as conformational changes in the alkyl group in the side chain. From the asynchronous 2D correlation spectrum (Fig. 3(b)),the FTIR band associated with the stretching N-H vibration of the amide group in the main chain of the surface immobilized PLL filmwas differentiated. The band assigned to the stretching vibration of the amide group was observed at 3200 cm-1 [26]. The signs of the cross peaks at (3270 and 3200),(3270 and 3030) and (3200 and 3030) cm-1 suggests that the decrease in protonation induced sequential spectral changes in the amino group in the side chain and the amide group in the main chain. The spectral changes in the peptide linkage in the main chain occur after the decrease in protonation. The signs of the cross peaks at (3200 and 2870) and (3030 and 2870) cm-1 suggest that the spectral changes in the alkyl group in the side chain occur after the decrease in protonation. Furthermore,the spectral changes in the peptide linkage in the main chain occur after those of the alkyl group in the side chain. Overall,these observations suggest that the decrease in protonation induces sequential spectral changes in the alkyl group of the side chain and the peptide linkage of themain chain. The spectral changes in the amino group and the alkyl group in the side chain occur before those of the peptide linkage in the main chain.

|
Download:
|
| Fig. 3. (a) Synchronous and (b) asynchronous 2D correlation spectra the region,3500-2650 cm-1,generated from the FTIR spectra of the surface-immobilized PLL film. The open and filled regions indicate positive and negative cross peaks,respectively. | |
The surface immobilized PLL film was examined in detail to provide pH-induced structural changes by FTIR spectroscopy. The decrease in the level of protonation of the side chain induced spectral changes in the amino group in the side chain and the peptide linkage in the main chain. In particular,the alkyl side group in the surface immobilized PLL film is strongly affected by pH changes. The alkyl side chain exhibited a more ordered conformation as the pH was increased. The stretching N-H vibration of the amide group was resolved by 2D FTIR correlation analysis. The 2D FTIR correlation spectra of the surface immobilized film with increasing pH suggested that a decrease in the protonation state of the amino group in the side chain induced spectral changes in the following sequence: amino group→alkyl chain→peptide unit.
AcknowledgmentsThis study was supported by Yeungnam University Research Grants in 2013 and a Human Resources Development Program of Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant (No. 20104010100580) funded by the Korean Ministry of Knowledge Economy.
| [1] | S. Edmondson, V.L. Osborne, W.T.S. Huck, Polymer brushes via surface-initiated polymerization, Chem. Soc. Rev. 33 (2004) 14-22. |
| [2] | M. Doebbelin, G. Arias, I. Loinaz, et al., Tuning Surface Wettability of poly (3-sulfopropyl methacrylate) brushes by cationic surfactantdriven interactions, Macromol. Rapid Commun. 29 (2008) 871-875. |
| [3] | O. Azzaroni, A.A. Brown, W.T.S. Huck, UCST wetting transitions of polyzwitterionic brushes driven by self-association, Angew. Chem. Int. Ed. 45 (2006) 1770-1774. |
| [4] | I. Borukhov, I. Leibler, Enthalpic stabilization of brush-coated particles in a polymer melt, Macromolecules 35 (2002) 5171-5182. |
| [5] | O. Azzaroni, Polymer brushes here, there, and everywhere: recent advances in their practical applications and emerging opportunities in multiple research fields, J. Polym. Sci. A: Polym. Chem. 50 (2012) 3225-3258. |
| [6] | C.M. Hui, J. Pietrasik, M. Schmitt, et al., Surface-initiated polymerization as an enabling tool for multifunctional (nano-) engineered hybrid materials, Chem. Mater. 26 (2014) 745-762. |
| [7] | R. Barbey, L. Lavanant, D. Paripovic, et al., Polymer brushes via surface-initiated controlled radical polymerization: synthesis, characterization, properties, and applications, Chem. Rev. 109 (2009) 5437-5527. |
| [8] | Y. Tsujii, K. Ohno, S. Yamamoto, A. Goto, T. Fukuda, Structure and properties of high-density polymer brushes prepared by surface-initiated living radical polymerization, Adv. Polym. Sci. 197 (2006) 1-45. |
| [9] | B. Zhao, W.J. Brittain, Polymer brushes: surface-immobilized macromolecules, J. Prog. Polym. Sci. 25 (2000) 677-710. |
| [10] | K. Matyjaszewski, P.J. Miller, N. Shukla, et al., Polymers at interfaces: using atom transfer radical polymerization in the controlled growth of homopolymers and block copolymers from silicon surfaces in the absence of untethered sacrificial initiator, Macromolecules 32 (1999) 8716-8724. |
| [11] | M. Baum, W.J. Brittain, Synthesis of polymer brushes on silicate substrates via reversible addition fragmentation chain transfer technique, Macromolecules 35 (2002) 610-615. |
| [12] | C.D. Grande, M.C. Tria, G. Jiang, R. Ponnapati, R. Advincula, Surface-grafted polymers from electropolymerized polythiophene RAFT agent, Macromolecules 44 (2011) 966-975. |
| [13] | A. Juang, O.A. Scherman, R.H. Grubbs, H. Robert, N.S. Lewis, Formation of covalently attached polymer overlayers on Si(1 1 1) surfaces using ring-opening metathesis polymerization methods, Langmuir 17 (2001) 1321-1323. |
| [14] | H.A. Haque, S. Kakehi, M. Hara, S. Nagano, T. Seki, High-density liquid-crystalline azobenzene polymer brush attained by surface-initiated ring-opening metathesis polymerization, Langmuir 29 (2013) 7571-7575. |
| [15] | B.J. Sparks, J.G. Ray, D.A. Savin, C.M. Stafford, D.L. Patton, Synthesis of thiolclickable and block copolypeptide brushes via nickel-mediated surface initiated polymerization of a-amino acid N-carboxyanhydrides (NCAs), Chem. Commun. 47 (2011) 6245-6247. |
| [16] | H. Duran, B. Yameen, H.U. Khan, R. Fö rch, W. Knoll, Surface-initiated ring opening polymerization of N-carboxy anhydride of benzyl-L-glutamate monomers on soft flexible substrates, React. Funct. Polym. 73 (2013) 606-612. |
| [17] | Y. Wang, Y.C. Chang, Preparation of unidirectional end-grafted a-helical polypeptides by solvent quenching, J. Am. Chem. Soc. 125 (2003) 6376-6377. |
| [18] | Y. Wang, Y.C. Chang, Synthesis and conformational transition of surface-tethered polypeptide: poly(L-lysine), Macromolecules 36 (2003) 6511-6518. |
| [19] | S.A. Curtin, T.J. Deming, Initiators for end-group functionalized polypeptides via tandem addition reactions, J. Am. Chem. Soc. 121 (1999) 7427-7428. |
| [20] | J. Wang, M.I. Gibson, R. Barbey, S.-J. Xiao, H.-A. Klok, Nonfouling polypeptide brushes via surface-initiated polymerization of N-oligo(ethylene glycol) succinate-L-lysine N-carboxyanhydride, Macromol. Rapid Commun. 30 (2009) 845-850. |
| [21] | R.J. Mart, R.D. Osborne, M.M. Stevens, R.V. Ulijn, Peptide-based stimuli-responsive biomaterials, Soft Matter 2 (2006) 822-835. |
| [22] | H. Block, Poly (Gamma-Benzyl-L-Glutamate) and Other Glutamic Acid Containing Polymers, Gordon & Breach Science Publishers, New York, 1983. |
| [23] | D. Kang, S.R. Ryu, Y. Park, B. Czarnik-Matusewicz, Y.M. Jung, pH-induced structural changes of ovalbumin studied by 2D correlation IR spectroscopy, J. Mol. Struct. 1069 (2014) 299-304. |
| [24] | W. Dzwolark, V. Smirnovas, A conformational a-helix to b-sheet transition accompanies racemic self-assembly of polylysine: an FTIR spectroscopic study, Biophys. Chem. 115 (2005) 49-54. |
| [25] | E.S. Manas, Z. Getahun, W.W. Wright, W.F. DeGrado, J.M. Vanderkooi, Infrared spectra of amide groups in a-helical proteins: evidence for hydrogen bonding between helices and water, J. Am. Chem. Soc. 122 (2000) 9883-9890. |
| [26] | M. Rozenberg, G. Shoham, FTIR spectra of solid poly-L-lysine in the stretching NH mode range, Biophys. Chem. 125 (2007) 166-171. |
| [27] | I. Noda, Generalized two-dimensional correlation method applicable to infrared, Raman, and other types of spectroscopy, Appl. Spectrosc. 47 (1993) 1329-1336. |
| [28] | B. Chae, S.W. Lee, M. Ree, Y.M. Jung, S.B. Kim, Photoreaction and molecular reorientation in a nanoscaled film of poly(methyl 4-(methacryloyloxy) cinnamate) studied by two-dimensional FTIR and UV correlation spectroscopy, Langmuir 19 (2003) 687-695. |
| [29] | Y. Ozaki, Kwansei Gakuin University, Sanda, Japan. http://science.kwansei.ac.jp/ ozaki/. |
| [30] | A. Dos, V. Schimming, S. Tosoni, H.-H. Limbach, Acid-base interactions and secondary structures of poly-L-lysine probed by 15N and 13C solid state NMR and Ab initio model calculations, J. Phys. Chem. B 112 (2008) 15604-15615. |
| [31] | Y.P. Myer, The pH-induced helix-coil transition of poly-L-lysine and poly-Lglutamic acid and the 238 mm dichroic band, Macromolecues 2 (1969) 624-628. |
| [32] | L. Sun, R.M. Crooks, A.J. Ricco, Molecular Interactions between organized, surfaceconfined monolayers and vapor-phase probe molecules. 5. Acid-base interactions, Langmuir 9 (1993) 1775-1780. |
| [33] | S.C. Yasui, T.A. Keiderling, Vibrational circular dichroism of polypeptides. 8. Poly(lysine) conformations as a function of pH in aqueous solution, J. Am. Chem. Soc. 108 (1986) 5576-5581. |




