Studies have shown the adverse health effects of particulate matters (PM) [1, 2]. Fine particles with an aerodynamic diameter less than 2.5mm(PM2.5) may be strongly related to respiratory diseases because these particles are small enough to reach conductive airways and the lower respiratory system [3]. In China,classroom teaching with boards and chalks,one of the cheapest methods of teaching,still dominates many parts of countryside. During chalk talk sessions,a large amount of chalk PM2.5are produced,which may be a menace to the teachers and students who are directly exposed to chalk dusts [4, 5]. However, little is known about their exact chemical compositions and toxic effects.
Chemiluminescence (CL) has been widely used as a sensitive and accurate method for the assessment of reactive oxygen species (ROS) production. Herein,after analyzing morphological and chemical compositions of fine chalk dust particles,we use a lumiol-dependent CL method to determine possible adverse effects of these particles on rat alveolar macrophages (AMs)in vitro. 2. Experimental
Chalk samples,which were made in Hubei Province,China,are available in the local market. White chalks were selected and grounded into powders using a mortar. Chalk PM2.5was collected on aluminum foils using a three-stage cascade Dekati PM10 impactor (Dekati Company,Finland),which has the aerodynamic cut-off diameters for the three stages of 10,2.5,and 1.0mm, respectively,at a sampling flow rate of 10 L/min. Fine chalk dust particles were collected and then numbered for analysis by the low-Z particle EPMA technique,which can simultaneously detect the morphology and constituent elements of an individual particle. Measurement was performed on a Zeiss Ultra 55 field-emission scanning electron microscopy (SEM) (Zeiss,Germany) equipped with an Oxford X-Max silicon drift detector (SDD). The size, secondary electron image (SEI),chemical compositions and the mixing state of chalk dust particles were investigated manually [6]. Silica (SiO2)PM2.5,used as a reference dust in the CL detection, was also obtained by the same method. AMs were obtained from the tracheal lavage of male Wistar rats (weighing 220-250 g) obtaining from the animal center of Hebei Medical University (Shijiazhuang,China) according to the method reported by Myrvik et al.[7]. The survived cells were tested by trypan blue staining. The cells with an average number of 2.4×10 6 per Petri dish (2 mL) were incubated for 2 h at 37℃ with 5% CO2 and above 95% humidity to allow AMs to adhere,then the cells were washed twice with 2 mL sterilized saline before incubation with particle suspensions at the doses of 50,100,200 or 500mg/10 6 cells for 4 h to perform biochemistry assays. The CL assay of the cells was performed as previously described [8]. Briefly,100mL of a luminol solution and 100mL of chalk PM2.5suspension were added to 1 mL of the cell suspension placed in a luminescence cuvette. After mixing,the CL was recorded by a luminescence analyzer. Light intensity was monitored continuously by a recorder and expressed in counts per sec (cps). 3. Results and discussion
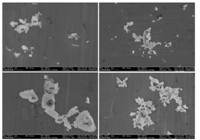
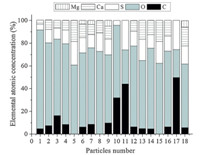
Earlier studies have reported the toxic effects of inorganic dusts such as silica and their relationship to ROS [9]. The components and toxicity of chalk dust,an inorganic dust,were uncertain. In our study,overall 172 particles were analyzed manually [10] and the typical morphology of chalk dust was illustrated (Fig. 1). The shape of the chalk particles appeared irregular with ambiguous borders. Based on their SEI data and EPMA results of chalk particles,oxygen, sulfur,calcium and carbon were found to be the main elements (Fig. 2). For CaSO4 and CaCO3 particles,the calculated atomic concentration ratios are [Ca]:[S]:[O]≈1:1:4 and [Ca]:[C]:[O]≈ 1:1:3,very close to their respective stoichiometry (Fig. 2), indicating the validity of the quantification procedure applied in this study. CaMg(CO3)2was analyzed by the same stoichiometry method. Excess C and O may be from organic adhesives. According to stoichiometry,particles #1-#4,#12-#14 and #16 consisted of calcium sulfate (CaSO4),calcite (CaCO3) and some organic adhesives. Particles #5,#8 and #15 were composed of only CaSO4. Particles #10,#11 and 17# consisted of CaSO4and organic adhesives,likely polyvinyl alcohol. And,(CaMg(CO3)2)andCaSO4 were found in particles #6,#7,#9 and #18. Our findings indicated that chalk dust was mainly composed of CaSO4,CaCO3/ CaMg(CO3)2 as well as organic adhesives such as polyvinyl alcohol. It is known that gypsum,which is a very soft mineral composed of calcium sulfate dihydrate,with the chemical formula CaSO4·2H2O,is generally used as crude material for chalk,thus it is not surprising that CaSO4was the representative components in chalk particles and content of CaSO4is high in chalk particles. It is necessary to add appropriate amount of CaCO3or CaMg(CO3)2to alter the hardness of chalk. Thus fine CaSO4,CaCO3/CaMg(CO3)2 particles can be produced when chalks are used. These particles may have adverse effects on AMs,which is supported by previous studies that fine gypsum or limestone dust,which is mainly composed of CaCO3,would exert adverse effects [11, 12, 13].
|
Download:
|
| Fig. 1. econdary electron images (SEIs) of typical chalk dust particles | |
|
Download:
|
| Fig. 2. Elemental concentrations in atomic fraction (at.%) and possible component for corresponding particles in Fig. 1. | |
It is well known that AMs are important target cells for particle interactions and deposition and therefore it is most likely that they can be affected by pollution particles. To assess whether chalk dust exposure impact the redox status of AMs,we evaluated oxidative response measured by luminol-dependent CL in AMs in response to chalk particle treatments. The results showed that CL of AMs reached a maximum value of about 830 cps when AMs were treated with 500μg chalk particles/10 6 cells,which was significantly higher than the effect of SiO2,suggesting that chalk PM2.5at high dose could have more significant effects than SiO2. In addition,respiratory burst was induced by chalk PM2.5 in a dose-dependent manner (Fig. 3A),which indicated that chalk PM2.5upon inhalation would be phagocytized by AMs and trigger the release of ROS,leading to pulmonary stress. CL of phagocytizing cells as macrophages is closely related to the production of reactive oxygen metabolites [14]. This is also confirmed by a similar study where exposure toa-quartz was found to increase oxidant production in AMs measured by lucigenin-enhanced CL [15]. The present study showed that chalk PM2.5exposure increased the oxidant production of AMs. Potential relevant targets include NADPH oxidase enzyme family members as well as mitochondria [16]. ROS include superoxide anion,hydroxyl radical,organic peroxide radicals,hydrogen peroxide,and singlet molecular oxygen. They are constantly generated intracellularly in eukaryotic cells upon exposure to different environmental stimuli [17]. Macrophage cells contain NADPH oxidase in their cytoplasmic membrane,which generates large amounts of O2· -in response to phagocytosis [18]. Among various inhibitors of oxygen radical generation, diphenyleneiodonium chloride (DPI,a flavoprotein inhibitor) targets the membrane NADPH oxidase complex and mitochondrial respiratory chain,while rotenone and antimycin target the mitochondrial respiratory chain. Effects of these inhibitors on chalk particle-induced activities in AMs were investigated here. A previous study found that a concentration less than 1μmol/L of DPI selectively inhibited the membrane NADPH oxidase complex [19]. We also obtained a similar result that the AM response to chalk particles was inhibited by about 90% by DPI but not affected by mitochondrial inhibitors (Fig. 3B), suggesting that chalk particles may be intimately involved in the NADPH dependent response to various microbes and microbial peptides.

|
Download:
|
| Fig. 3. Chemiluminescence effect of AMs exposed to different doses of chalk PM2.5(A) and inhibition by oxygen radical inhibitors rotenone (2.5μmol/L),antimycin (2.5μmol/L), and DPI (0.5μmol/L) for 30 min before the measure of CL (B). The bars present mean±SD of six experiments. SiO2PM2.5was used as a reference. Significant difference is indicated by *p<0.05 and ***p<0.001 | |
Single-particle analysis using low-ZEPMA showed that chalk particles were mainly composed of CaSO4,CaCO3/CaMg(CO3)2, and organic adhesives. Fine chalk dust particles could stimulate AMs to produce CL in a dose-independent manner. The data showed that cytotoxicity of chalk PM2.5may be related to the ROS generation.
Acknowledgment This research was supported by grants from the National Natural Science Foundation of China (Nos. 21107064,2140509, 21175086 and 21177078) and by grant from Shanxi Scholarship Council of China (Nos. 2013-012 and 2013-016).| [1] | P.C. Zeidler, A. Hubbs, L. Battelli, V. Castranova, Role of inducible nitric oxide synthase-derived nitric oxide in silica-induced pulmonary inflammation and fibrosis, J. Toxicol. Environ. Health A 67 (2004) 1001-1026. |
| [2] | R.J. Li, X.J. Kou, H. Geng, C. Dong, Z.W. Cai, Pollution characteristics of ambient PM2.5-bound PAHs and NPAHs in a typical winter time period in Taiyuan, Chin. Chem. Lett. 25 (2014) 663-666. |
| [3] | E. Bastonini, L. Verdone, S. Morrone, et al., Transcriptional modulation of a human monocytic cell line exposed to PM10 from an urban area, Environ. Res. 111 (2011) 765-774. |
| [4] | D. Majumdar, S.P.M. Prince William, Chalk dust fall during classroom teaching: particle size distribution and morphological characteristics, Environ. Monit. Assess. 148 (2009) 343-351. |
| [5] | S. Weichenthal, A. Dufresne, C. Infante-Rivard, L. Joseph, Characterizing and predicting ultrafine particle counts in Canadian classrooms during the winter months: model development and evaluation, Environ. Res. 106 (2008) 349-360. |
| [6] | H. Geng, J.Y. Ryu, S. Maskey, H.J. Jung, C.U. Ro, Characterisation of individual aerosol particles collected during a haze episode in Incheon, Korea using the quantitative ED-EPMA technique, Atmos. Chem. Phys. 11 (2011) 1327-1337. |
| [7] | Q.N. Myrvik, E.S. Leake, B. Fariss, Lysozyme content of alveolar and peritoneal macrophages from the rabbit, J. Immunol. 86 (1961) 133-136. |
| [8] | H. Geng, Z.Q. Meng, Inhibition of superoxide dismutase, vitamin C and glutathione on chemiluminescence produced by luminol and the mixture of sulfite and bisulfate, Spectrochim. Acta A 64 (2006) 87-92. |
| [9] | T.T. Chen, Y.H. Hu, Y. Cen, X. Chu, Y. Lu, A dual-emission fluorescent nanocomplex of gold-cluster-decorated silica particles for live cell imaging of highly reactive oxygen species, J. Am. Chem. Soc. 135 (2013) 11595-11602. |
| [10] | C.U. Ro, J. Osá n, I. Szalóki, et al., A Monte Carlo program for quantitative electron-induced X-ray analysis of individual particles, Anal. Chem. 75 (2003) 851-859. |
| [11] | A. Clouter, C.E. Houghton, L.R. Hibbs, et al., Effect of inhalation of low doses of crocidolite and fibrous gypsum on the glutathione concentration and g-glutamyl transpeptidase activity in macrophages and bronchoalveolar lavage fluid, Inhal. Toxicol. 10 (1998) 3-14. |
| [12] | E.F.M. Wouters, T.H.J.M. Jorna, M. Westenend, Respiratory effects of coal dust exposure: clinical effects and diagnosis, Exp. Lung Res. 20 (1994) 385-394. |
| [13] | F. Crummy, I. Carl, C.H.S. Cameron, et al., A possible case of pneumoconiosis in a limestone quarry worker, Occup. Med. 54 (2004) 497-499. |
| [14] | N.A. Paterson, D.J. McIver, S. Schurch, The effect of leukotrienes on porcine alveolar macrophage function, Prostaglandins Leukot. Med. 25 (1986) 147-161. |
| [15] | Z. Zhang, H.M. Shen, Q.F. Zhang, C.N. Ong, Involvement of oxidative stress in crystalline silica induced cytotoxicity and genotoxicity in rat alveolar macrophages, Environ. Res. 82 (2000) 245-252. |
| [16] | A.M. Scherbart, J. Langer, A. Bushmelev, et al., Contrasting macrophage activation by fine and ultrafine titanium dioxide particles is associated with different uptake mechanisms, Part. Fibre Toxicol. 8 (2011) 31-49. |
| [17] | B. Halliwell, J.M.C. Gutteridge, Free Radicals in Biology and Medicine, Claredon Press, Oxford, 1989. |
| [18] | D. Breznan, P. Goegan, V. Chauhan, et al., Respiratory burst in alveolar macrophages exposed to urban particles is not a predictor of cytotoxicity, Toxicol. In Vitro 27 (2013) 1287-1297. |
| [19] | Y. Li, M.A. Trush, Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production, Biochem. Biophys. Res. Commun. 253 (1998) 295-299.. |




