b Centre of Drug Discovery, China Pharmaceutical University, Nanjing 210009, China
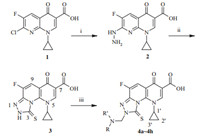
Currently,fluoroquinolones as broad-spectrum antimicrobial agents are the most attractive drugs in the anti-infective chemotherapy field besidesb-lactam and macrolide antibiotics [1]. However,the emergence of bacterial resistance to the quinolones is a growing health problem in the community and hospital settings,and the need for safe and affordable antibacterial agents capable of overcoming the resistance is urgent [2]. Several strategies overcome bacterial resistance being executed and the most effective strategy appears to continually modify the existing classes of antibacterial agents to provide new analogues [3]. The structure-activity relationship (SAR) of fluoroquinolones showed that the fluorine atom and the 1-alkyl,1,4-dihydro-4-oxoquinoline-3-carboxylic acid skeleton are responsible for potency in binding with type-II topoisomerase enzymes,DNA gyrase and topoisomerase IV [4]. Moreover,it is believed that the 6-fluoro and 7-piperazinyl groups are responsible for the broad spectrum of fluoroquinolones [5]. Furthermore,it is clear that chemical modifications at C-7 are suitable for controlling of the pharmacokinetic properties and enhancing the cell permeability [6]. Consequently,many contributions mainly focused on the substituent changes at the N1- and 7-positions [7] or formation of fused heterocyclic ring between the N1- and 8-positions [8],whereas about the effects of an azole heterocyclic ring as a moiety of tricycle-fused quinoline-3-carboxylic acid skeleton between the 7-and 8-positions are less known.s-Triazole is a class of heterocycles with a wide range of applications that are receiving considerable attention [9].s-Triazole and its Mannich-base derivatives exhibited various biological activities as antibacterial,antitumor,antiinflammatory and antifungal agents [10]. Additionally,previously we have synthesized tetra-cyclic fluoroquinolone carboxylic acid derivatives with an s-triazole ring fused on the N-1 and 8-positions,which showed potent antibacterial activity [11]. In continuation of our ongoing anti-infective research project,an attempt to incorporate the triazole with a functional Mannich-base chain into the 7- and 8-positions of the fluoroquinolone scaffold led to novel tricyclic fluoroquinolone-7-carboxylic acid derivatives 4a-4h(Scheme 1).
|
Download:
|
| Scheme 1.Synthetic route for the titled compounds 4a-4h. Reagents and conditions: (i) 80% hydrazine hydrate,DMSO,50℃; (ii) CS2,Et3N,DMF,r.t. to reflux; (iii) amines,37% formaldehyde,MeOH,r.t. Amine donors: dimethylamine (4a); diethylamine (4b); piperidine (4c); morpholine (4d); N-methyl piperazine (4e);N-ethyl piperazine (4f); 2-methylpiperazine (4g); pyrrolidine (4h/b>) | |
Melting points were measured on a XRD micro melting point apparatus and uncorrected. 1H NMR spectra were recorded on a Bruker AM-400 spectrometer using TMS as an internal reference. Mass spectra were recorded on a Bruker Esquire-LC instrument. Elemental analysis was carried out using a PE2400 II instrument. All reagents and solvents purchased from commercial vendors and were used without further purifications.
1-Cyclopropyl-6-fluoro-7-hydrazino-[1, 8]naphthyridin-4(1H)-one-3-carboxylic acid(2): A suspension of 1(20.0 g,70.8 mmol) and hydrazine monohydrate (7.0 g,140.0 mmol) in DMSO (100 mL) was stirred at 50℃ for 12 h. After cooling to room temperature,the precipitates formed were collected by filtration and washed with water. The crude product 2 was dissolved in dilute aqueous hydrochloric acid (3.6%,200 mL),adequate amount of active charcoal was added and decolorized at 60℃ for 1 h,and the filtrate was neutralized to pH 7.0. The resulting precipitates were collected and washed with water to afford 2(14.7 g,75.0%) as yellow solid,mp 232-234℃; MS (m/z): 279 [M+H]+ ; Calcd. for C12H11FN4O3: 278.24.
5-Cyclopropyl-10-fluoro-3-thioxo-2,3-dihydro-[1, 2, 4]triazolo[3, 4, h][1, 8]naphthyridine-7-carboxylic acid(3): A mixture of hydrazino compounds 2(10.0 g,36.0 mmol) and carbon disulfide (4.1 g,54.0 mmol) in DMF (100 mL) was refluxed for 10 h. The reaction mixture was poured into ice-water (100 mL). The precipitates were collected by filtration,washed with water and dried. The resulting crude 3 was recrystallized from a mixed solvent of EtOH-DMF to obtain compound 3 in 68% yield,mp 253- 255℃. 1H NMR (400 MHz,DMSO-d6): δ15.36 (brs,1H,COOH), 13.75 (s,1H,NH or SH),8.87 (s,1H,6-H),7.84 (d,J= 13.2 Hz,1H,9-H),3.67-3.53 (m,1H,1´ -H),1.27-1.23 (m,4H,2´ - and 3´ -H); MS (m/ z): Calcd. for C13H9FN4O3S: 320.30 M+ ,found: 321 [M+H]+ ; Anal. Calcd. for C13H9FN4O3S: C 48.75,H 2.83,N 17.49; Found: C 48.96,H 2.68,N 17.73.
1-Amino-5-cyclopropyl-10-fluoro-3-thioxo-2,3-dihydro-[1, 2, 4]triazolo[3, 4, h][1, 8]naphthyridine-7-carboxylic acids (4a- 4h): To a solution of compound 3(0.5 g,1.6 mmol) in absolute ethanol (10 mL) was added the appropriate amine donors (1.6 mmol) followed by a 37% formaldehyde solution (0.5 mL). The mixture was refluxed for 24 h. After the removal of the solvent, the solid residue was dissolved in dilute aqueous hydrochloric acid. The filtrate was neutralized to pH 7.0 with NaHCO3 saturated solution followed by an extraction with CHCl3. The organic layer was dried under anhydrous Na2SO4 and concentrated under reduced pressure to give the crude product,which was recrystallized from anhydrous ethanol to give the targeted compounds 4a-4h.
Compound 4a: Yield 68%,mp 214-216℃; 1H NMR (400 MHz, DMSO-d6): δ15.43 (brs,1H,COOH),8.86 (s,1H,6-H),7.86 (d, J= 13.2 Hz,1H,9-H),5.18 (s,2H,NCH2),3.63-3.54 (m,1H,1´-H), 2.43 (s,6H,2×CH3),1.26-1.21 (m,4H,2´- and 3´-H); MS (m/z): Calcd. for C16H16FN5O3S: 377.40 M+ ,found: 378 [M+H]+ ; Anal. Calcd. forC16H16FN5O3S: C 50.92,H 4.27,N 18.56; Found: C 51.12, H 4.38,N 18.74.
Compound 4b: Yield 54%,mp 202-204℃; 1H NMR (400 MHz, DMSO-d6): δ15.44 (brs,1H,COOH),8.87 (s,1H,6-H),7.83 (d, J= 13.2 Hz,1H,9-H),5.16 (s,2H,NCH2),3.66-3.54 (m,1H,1´-H), 2.42-2.48 (m,4H,2×CH2),1.27-1.13 (m,10H,2´-,3´-H and 2× CH3); MS (m/z): Calcd. for C18H20FN5O3S: 405.45 M+ ,found: 406 [M+H]+ ; Anal. Calcd. for C18H20FN5O3S: C 53.32,H 4.97,N 17.27; Found: C 53.52,H 4.74,N 17.53.
Compound 4c: Yield 48%,mp 228-230℃; 1H NMR (400 MHz, DMSO-d6): δ15.38 (brs,1H,COOH),8.83 (s,1H,6-H),7.82 (d, J= 13.2 Hz,1H,9-H),5.08 (s,2H,NCH2N),3.62-3.55 (m,1H,1´ -H), 2.28-3.04 (m,4H,N(CH2)2),1.54-1.06 (m,10H,2´-,3´-H and 3× CH2); MS (m/z): Calcd. for C19H20FN5O3S: 417.47 M+ ,found: 418 [M+H]+ ; Anal. Calcd. for C19H20FN5O3S: C 54.67,H 4.83,N 16.78; Found: C 54.86,H 4.68,N 16.94.
Compound 4d: Yield 63%,mp 236-238℃; 1H NMR (400 MHz, DMSO-d6): δ15.44 (brs,1H,COOH),8.87 (s,1H,6-H),7.85 (d, J= 13.2 Hz,1H,9-H),5.22 (s,2H,NCH2N),3.64-3.58 (m,5H,10-H and O(CH2)2),3.26 (t,J= 5.4 Hz,4H,N(CH2)2),1.31-1.25 (m,4H,2´ -and 3´ -H); MS (m/z): Calcd. for C18H18FN5O4S: 419.44 M+ ,found: 420 [M+H]+ ; Anal. Calcd. for C18H18FN5O4S: C 51.55,H 4.33,N 16.70; Found: C 51.76,H 4.12,N 16.87.
Compound 4e: Yield 73%,mp 237-239℃; 1H NMR (400 MHz, DMSO-d6): δ15.46 (brs,1H,COOH),8.89 (s,1H,6-H),7.86 (d, J= 13.2 Hz,1H,9-H),5.23 (s,2H,NCH2N),3.66-3.37 (m,5H,10-H and N(CH2)2),2.68-2.54 (m,4H,N(CH2)2),2.27 (s,3H,N-CH3), 1.33-1.21 (m,4H,2´ - and 3´ -H); MS (m/z): Calcd. for C19H21FN6O3S: 432.48 M+ ,found: 433 [M+H]+ ; Anal. Calcd. for C19H21FN6O3S: C 52.77,H 4.89,N 19.43; Found: C 52.98,H 4.76,N 19.68.
Compound 4f: Yield 63%,mp 231-233℃; 1H NMR (400 MHz, DMSO-d6): δ15.45 (brs,1H,COOH),8.88 (s,1H,6-H),7.84 (d, J= 13.2 Hz,1H,9-H),5.21 (s,2H,NCH2N),3.68-3.35 (m,5H,1 ´-H and N(CH2)2),2.66-2.57 (m,4H,N(CH2)2),2.24 (q,J=5.6 Hz,2H, NCH2CH3),1.37-1.22 (m,7H,CH3,2´- and 3´ -H); MS (m/z): Calcd. for C20H23FN6O3S: 446.51 M+ ,found: 447 [M+H]+ ; Anal. Calcd. for C20H23FN6O3S: C 53.80,H 5.19,N 18.82; Found: C 53.97,H 5.36,N 18.71.
Compound 4g: Yield 51%,mp 207-209℃; 1H NMR (400 MHz, DMSO-d6): δ15.43 (brs,1H,COOH),8.87 (s,1H,6-H),7.85 (d, J= 13.2 Hz,1H,9-H),5.17 (s,2H,NCH2N),3.66-3.35 (m,5H,10-H and N(CH2)2),2.63-2.54 (m,3H,CHNCH2),1.36-1.18 (m,7H,CH3, 2´- and 3´ -H); MS (m/z): Calcd. for C19H21FN6O3S: 432.48 M+, found: 433 [M+H]+ ; Anal. Calcd. for C19H21FN6O3S: C 52.77,H 4.89, N 19.43; Found: C 52.62,H 4.73,N 19.64.
Compound 4h: Yield 78%,mp 236-238℃; 1H NMR (400 MHz, DMSO-d6): δ15.36 (brs,1H,COOH),8.85 (s,1H,6-H),7.82 (d, J= 13.2 Hz,1H,9-H),5.13 (s,2H,NCH2N),3.61-3.54 (m,1H,1´-H), 3.04-2.78 (m,4H,N(CH2)2),1.58-1.06 (m,8H,CH2CH2,2 ´- and 3´ -H); MS (m/z): Calcd. for C18H18FN5O3S: 403.44 M+ ,found: 404 [M+H]+ ; Anal. Calcd. for C18H18FN5O3S: C 53.59,H 4.50,N 17.36; Found: C 53.82,H 4.38,N 17.62. 3. Results and discussion
The antibacterial activity of the titled compounds was screened using the microdilution method against Gram-positiveStaphylococcus aureusATCC-29213,Methicillin-resistantS. aureusATCC-25923,Gram-positive E. coli ATCC-29213,and Multiple drugresistantE. coliATCC-35218 bacterial strains using ciprofloxacin (Cipro.) as a comparator. The results are presented in Table 1. A structure-activity relationship analysis revealed that the Mannich-base-containing compounds (4c-4h) derived from cycloaliphatic amine donors are more potent than those derived from acyclic aliphatic amines such as compounds (4a-4c). Moreover, compounds carrying both piperazine and pyrrolidine Mannichbase moieties displayed better activity than both piperidine (4c) and morpholine (4d) compounds against standard bacterial strains or drug-resistant strains. Interestingly,except for piperazine Mannich-base derivatives,especially 2-methylpiperazine compound (4h) were highly active against Multiple drug-resistantE. coliATCC-35218 bacterial strain with an MIC value of 0.25mg/mL, about 30-fold more potent than ciprofloxacin.
| Table 1 Inhibitory activity (MIC,mg/μL) of the synthetic compounds against microbes. |
In summary,eight novels-triazole-fused tricyclic fluoroquinolone carboxylic acid derivatives (4a-4h) were synthesized and evaluated for their in vitro antibacterial activity. Some of compounds carrying piperazine or pyrrolidine Mannich-base moiety showed potent activity against all tested bacterial strains. In particular,the methylpiperazine compound (4h) exhibited about 8- to 30-fold more potent activity against the drug-resistant strains than ciprofloxacin. These results demonstrated that triazole-fused fluoroquinolones could be promising lead compounds in discovery of novel anti-infective chemotherapies.
AcknowledgmentWe gratefully acknowledge the generous support provided by the National Natural Science Foundation of China (Nos. 20872028 and 21072045)。 and 21072045)。
| [1] | T. Tomašić, L.P. Mašič, Prospects for developing new antibacterials targeting bacterial type IIA topoisomerases, Curr. Top. Med. Chem. 14 (2014) 130-151. |
| [2] | X.D. Wang, W. Wei, P.F. Wang, et al., Novel 3-arylfuran-2(5H)-one-fluoroquinolone hybrid: design, synthesis and evaluation as antibacterial agent, Bioorg. Med. Chem. 22 (2014) 3620-3628. |
| [3] | K. Chauhan, P. Singh, V. Kumar, et al., Investigation of Ugi-4CC derived 1Htetrazol- 5-yl-(aryl) methyl piperazinyl-6-fluoro-4-oxo-1 4-dihydroquinoline-3- carboxylic acid: synthesis, biology and 3D-QSAR analysis, J. Eur. Med. Chem. 78 (2014) 442-454. |
| [4] | K.M. Hutchings, T.P. Tran, E.L. Ellsworth, et al., Synthesis and antibacterial activity of the C-7 side chain of 3-aminoquinazoline-diones, Bioorg. Med. Chem. Lett. 18 (2008) 5087-5090. |
| [5] | M.J. Nieto, A.B. Pierini, N. Singh, et al., SAR analysis of new dual targeting fluoroquinolones. Implications of the benzenesulfonyl group, Med. Chem. 8 (2012) 349-360. |
| [6] | H. Takiff, E. Guerrero, Current prospects for the fluoroquinolones as first-line tuberculosis therapy, Antimicrob. Agents Chemother. 55 (2011) 5421-5429. |
| [7] | C. Zhi, Z.Y. Long, A. Manikowski, et al., Hybrid antibacterials. DNA polymerase- topoisomerase inhibitors, J. Med. Chem. 49 (2006) 1455-1465. |
| [8] | Y. Jinbo, H. Kondo, M. Taguchi, et al., Synthesis and antibacterial activity of thiazolopyrazine-incorporated tetracyclic quinolone antibacterial agents. 2, J. Med. Chem. 37 (1994) 2791-2796. |
| [9] | Y.D. Li, W.T. Mao, Z.J. Fan, et al., Synthesis and biological evaluation of novel 1,2,4- triazole containing 1,2,3-thiadiazole derivatives, Chin. Chem. Lett. 24 (2013) 1134-1136. |
| [10] | M. Koparir, C. Orek, A.E. Parlak, et al., Synthesis and biological activities of some novel aminomethyl derivatives of 4-substituted-5-(2-thienyl)-2,4-dihydro-3H- 1,2,4-triazole-3-thiones, J. Eur. Med. Chem. 63 (2013) 340-346. |
| [11] | G.Q. Hu, Z.Q. Zhang, W.L. Huang, H.B. Huang, S.T. Huang, Synthesis and antibacterial activity of new tetracyclic triazolothiadiazino fluoroquinolones, Chin. Chem. Lett. 15 (2004) 23-25. |





