The intense interest in the synthesis of coordinately unsaturated transition metal complexes arises from the essential role that such compounds play as key intermediates in significant catalytic processes [1, 2, 3]. In the context of catalytic epoxidation,C-H bond activation and functionalization,d6 and d8 electron deficient metal complexes (14 and 16-electron) are of great significance. In recent years a number of electron deficient transition metal complexes with 14 [4, 5, 6, 7, 8] and even 12 valence electrons [9, 10, 11] have been prepared and characterized. A survey of relevant literature shows that a key strategy in successful preparation of the electron deficient species is the application of bulky ligands to sterically inhibit the addition of electron donating groups. Several studies have been performed on the synthesis and properties of disilene electron deficient complexes [10, 12, 13]. Berry et al. reported disilene complexes of Cp2W(Si2Me4). Kira and co-workers reported several 12,14 and 16-electron disilene Pt(II) and Pd(II) complexes [9, 10]. Nolan’s research group discovered a 14-electron Rh(III) complex that was active in C-H bond activation [5]. Van der Schaaf and co-workers synthesized a 14-electron Ru(II) benzylidene complex (the Grubbs’ catalyst) [6, 7]. Nevertheless,electron deficient complexes of transition metals have been ignored so far. To the best of our knowledge,no electron deficient coordination polymer has been reported. Since such unsaturated compounds can be key intermediates in catalytic olefin epoxidation reactions [14, 15, 16],their isolation and characterization remain a necessary goal. Electron deficient species have been proposed as the key intermediates in the catalytic olefin epoxidation and the asymmetric epoxidation of olefins [15] is a very important organic transformation since the resulting enantiomerically pure epoxides are highly useful intermediates and building blocks. Several chiral catalysts that can carry out an asymmetric epoxidation reaction effectively have been developed. The most successful examples are the chiral Ru(II) and Ru(III) complexes. Belleret al.reported a high yielding and moderately enantioselective epoxidation reaction of styrenes using a chiral Ru(II) complex as catalysts to produce styrene oxides [17]. Kureshy’s research group has revealed that chiral ruthenium Schiff base complexes could improve the enantiomeric excess of the epoxidation products [18, 19]. Subsequently,Katsuki and coworkers described ruthenium(III) complexes in combination with 2,6-dichloropyridine N-oxide (DCPNO) or tetramethylpyrazine N,N´ -dioxide as oxidant act as an efficient epoxidation catalyst [20]. Ideally,more efforts should be devoted to these catalytic systems. As a part of current interest in our research group on the oxidation reactions,we describe here the synthesis,crystal structure and catalytic activity of an unprecedented polymeric electron deficient ruthenium(III) complex for the epoxidation of styrene and its derivatives with tert-butyl hydrogen peroxide,TBHP,as an oxidant. The selective epoxidation of styrenes was performed with excellent yields. 2. Experimental
All chemicals and reagents were purchased from Aldrich Strem chemical companies. Single-crystal X-ray diffraction data were collected on a STOE IPDS-II diffractometer with graphite monochromated Mo Ka radiation at room temperature using the Stoe X-AREA software [21]. The structure was solved using STR-92 [22] and refined using the full-matrix least-squares methods onF2 , within SHELXTL V6.1 [23]. For monitoring of reaction products and their identity,a gas chromatograph,Agilent Technologies 7890A Instrument equipped with a HP-1 capillary column,a FID detector, and a mass spectroscope model 5975C with a triple-axis detector was used. Elemental analysis was performed using a Heraeus CHN-O Rapid analyzer. The determination of ruthenium was performed on a Varian model VISTA-PRO inductively plasma optical emission spectrometer (ICP-OES).
Preparation of Ru complex (1): A solution of RuCl2(PPh3)3 (100 mg,0.104 mmol) in CHCl3 (10 mL) was stirred in the presence of air at ambient temperature,then a solution of triazine (16 mg,0.208 mmol) in acetone (10 mL) was added. The color of the reaction changed from dark brown to deep blue immediately. After filtration of reaction mixture over celite,diethyl ether (15 mL) was added. Partial evaporation of the solvents yielded a brown precipitate. Recrystallization from 10 mL of the 2:1 MeCN/MeOH mixture gave yellow microcrystals after 2 days (yield 63%). Elemental analysis: Calcd. for [Ru(HCO2)Cl2]: C,5.53; H,0.46; Cl,32.72; Ru,46.54. Found: C,5.48; H,0.49; Cl,32.62; Ru,46.33.
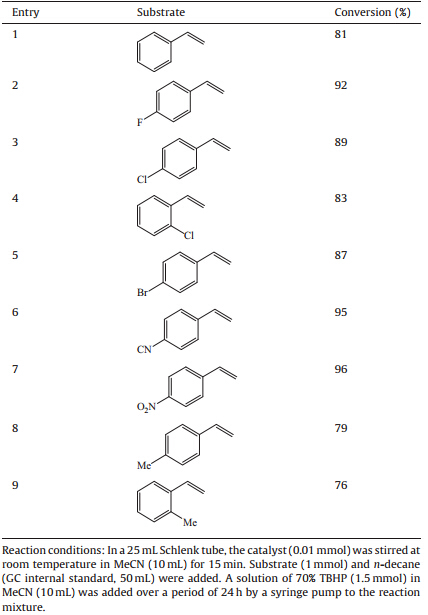
Catalytic epoxidation: In a 25 mL Schlenk tube,the catalyst (0.01 mmol) was stirred at room temperature in MeCN (10 mL) for 15 min. Substrate (1 mmol) andn-decane (GC internal standard, 50 mL) were added. A solution of 70% TBHP (1.5 mmol) in MeCN (10 mL) was added over a period of 24 h by a syringe pump to the reaction mixture. The reaction products were monitored by gas chromatography. 3. Results and discussion
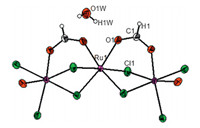
The result of single crystal X-ray diffraction of the prepared crystals is shown in Table 1. The structure crystallizes in a chiral hexagonal space group P6122 with six molecules in the unit cell. The asymmetric unit contains one Ru(III) ion,the half of a formate ligand and one chloride anion. Each ruthenium atom is sixcoordinated,comprising four bridging chloride anions and two oxygen atoms of two formate groups bridged between ruthenium centers (Fig. 1).
| Table 1 Crystal data and structure refinement for [Ru(HCO2)Cl2]n. |
|
Download:
|
| Fig. 1. Molecular structure of 1 with 50% probability of thermal ellipsoids. Symmetry independent part is labeled. | |
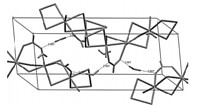
The coordination environment of ruthenium is best described as distorted octahedral,in which the axial positions are occupied by one oxygen atom from the formate ligand and one bridging chloride ligand. The equatorial plane involves another oxygen atom of chelating formate together with three bridging chlorides. Packing diagram of the structure is shown in Fig. 2. Expansion of the structure leads to the formation of an infinite 1-D chain coordination polymer that could be formulated as [Ru(HCO2)Cl2]n (Fig. 3).
|
Download:
|
| Fig. 2. Packing diagram of [Ru(HCO2)Cl2]n. | |

|
Download:
|
| Fig. 3. The perspective view of 1-D chains of the structure. | |
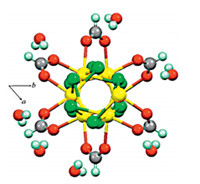
The chelation of the carboxylate O atoms in the formate groups in [Ru(HCO2)Cl2]nshows thesyn,syncoordination fashion [Ru-O bond distances = 2.304(5) Å]. As reported previously [24],carboxylate anions bridged between two identical metal coresviaboth oxygen atoms in μ2-η 2 fashion can adoptanti,anti;syn,antiandsyn,syn manner for the formate ion unless the metal atoms are bound together or connected by other bridging ligands. In the latter cases, only thesyn,synmode is possible. Extension of the structure through the formate ligands and the crystallographic symmetry generates 6-fold homochiral right-handed (Δ) [Ru(HCO2)Cl2]n helix that is oriented along thecdirection (Fig. 4)
|
Download:
|
| Fig. 4. Showing the 6-fold homochiral helix along crystallographiccaxis. | |
Crystallographic data,selected bond lengths (Å ) and angles [8] of [Ru(HCO2)Cl2]nare depicted in Tables 1 and 2.
| Table 2 Bond lengths [Å ] and angles [8] for [Ru(HCO2)Cl2]n. |
The Ru...Ru distances are approximately 3.604 Å ,significantly longer than the bond length expected for the corresponding metal- metal bonds [25]. Bridging the formate groups between the two metal centers makes a torsion angle of around 7.90°. There are also hydrogen bonds between the O-H of the free water molecules and oxygen of the formate groups (O(1)W...O(1) = 2.849(6) Å and H(1)W...O1 = 2.007(6)). The observed hydrogen bonds can further stabilize the whole structure.
This coordination polymer was prepared by simple treatment of a mixture of RuCl2(PPh3)3in chloroform and triazine in acetone at 25℃. The origin of formate ligands in the structure is a well known reaction between acetone and chloroform. Under basic conditions chloroform can be hydrolyzed according to the following mechanism (Scheme 1) and formate ions were produced [26].
suggests that the Ru atoms in this molecule possess only 13 valence electrons for the central Ru(III) ion with five valence electrons, four electrons from the oxygen atoms in the formate ions and four electrons from the bridging chloride ions. But if we consider bridging chlorides as four electron donors,the complex would have 17 valence electrons. Without further theoretical calculations or instrumental experiments we cannot decide for number of valence electrons.
In preliminary studies,we evaluated the catalytic property of the [Ru(HCO2)Cl2]nin the epoxidation of styrene with TBHP as an oxidant (Table 3).
This electron deficient Ru(III) complex,[Ru(HCO2)Cl2]n,was employed in the epoxidation of para-fluorostyrene,affording the styrene oxide in 92% yield (entry 2).Para-chlorostyrene was then selected as a model substrate and 89% yield was observed (entry 3). These data exhibit great enhancement in reactivity in comparison with the results obtained by the other systems reported previously [21]. After these introductory catalytic results,epoxidation of a variety of functionalized styrenes were then examined using the polymeric Ru(III) complex with TBHP oxidant at ambient temperature. Noticeably,the substituted groups of different electronic characters on the phenyl ring of the styrenes influenced the reactivity. For all of the tested styrenes,good yields were obtained,and in general,substituted styrenes with electron withdrawing groups such aspara-fluoro, para-nitro and ortho-chloro were epoxidized with higher rate. As summarized in Table 3,the reactions carried on efficiently to give the corresponding styrene oxides in high yields. All evidence suggests that the novel Ru(III) coordination polymer showed high activity in the catalytic epoxidation of styrenes. By comparison of complex [Ru(HCO2)Cl2]nand other catalysts with Ru as a metal core,it is clear that electron deficiency is crucial to reach high catalytic activity.
| Table 3 Epoxidation of styrenes catalyzed by complex [Ru(HCO2)Cl2]n. |
We have successfully synthesized and characterized an unprecedented chiral Ru(III) complex of simple chloride and formate ligands by mixing of RuCl2(PPh3)3,in a chloroform- acetone mixture,as a source of formate anion,in the presence of triazine as an organic base. This electron deficient coordination complex,[Ru(HCO2)Cl2]n,catalyzes rapid,efficient and selective epoxidation of various styrenes under mild reaction conditions. Because of the natural chirality,this coordination polymer can be used as a catalyst in asymmetric reactions. Further studies focusing on the development of the scope of this catalytic system for asymmetric epoxidation as well as the improvement of more efficient analogous catalysts are underway.
Supplementary materialFull crystallographic details are deposited with the Cambridge Structural Database Centre (CCDC No. 822322). These data can be obtained free of charge on application to CCDC,12 Union Road,Cambridge CB2 1EZ,UK (fax: +44 1223 336 033; E-mail: deposit@ccdc.cam.ac.uk).
AcknowledgmentThe authors gratefully acknowledge the financial support from the University of Tehran.
| [1] | H. Ogino, Synthesis of silylene and silyl(silylene)metal complexes, Chem. Rec. 2 (2002) 291-306. |
| [2] | X. Zhang, A. Fried, S. Knapp, A.S. Goldman, Novel synthesis of enamines by iridium-catalyzed dehydrogenation of tertiary amines, Chem. Commun. (2003) 2060-2061. |
| [3] | U. Christmann, R. Vilar, Monoligated palladium species as catalysts in crosscoupling reactions, Angew. Chem. Int. Ed. 44 (2005) 366-374. |
| [4] | R. Dorta, R. Goikhman, D. Milstein, Reactivity of [Ir(COE)2(solvent)2]PF6 complexes toward alkylphosphines: room-temperature C-H activation (cyclometalation) and isolation of a 14-electron alkyl-iridium(Ⅲ) complex, Organometallics 22 (2003) 2806-2809. |
| [5] | P.A. van der Schaaf, R. Kollya, A. Hafner, A 14-electron ruthenium hydride: the key intermediate in the synthesis of ruthenium carbene complexes; X-ray structure of[RuHCl(PPr3i)2], Chem. Commun. (2000) 1045-1046. |
| [6] | C.S. Yi, D.W. Lee, Z.J. He, Acid-promoted homogeneous hydrogenation of alkenes catalyzed by the ruthenium-hydride complex (PCy3)2(CO)(Cl)RuH: evidence for the formation of 14-electron species from the selective entrapment of the phosphine ligand, Organometallics 19 (2000) 2909-2915. |
| [7] | A.C. Sykes, P. White, M. Brookhart, Reactions of anilines and benzamides with a 14-electron iridium(I) bis(phosphinite) complex: N-H oxidative addition versus Lewis base coordination, Organometallics 25 (2006) 1664-1675. |
| [8] | C. Watanabe, T. Iwamoto, C. Kabuto, M. Kira, Fourteen-electron bis(dialkylsilylene) palladium and twelve-electron bis(dialkylsilyl)palladium complexes, Angew. Chem. Int. 47 (2008) 5386-5389. |
| [9] | H. Hashimoto, Y. Sekiguchi, T. Iwamoto, C. Kabuto, M. Kira, Synthesis and X-ray structure of a platinum η2-disilene complex, Organometallics 21 (2002) 454-456. |
| [10] | A.J. Schultz, J.M. Williams, R.R. Schrock, G.A. Rupprecht, J.D. Fellmann, Interaction of hydrogen and hydrocarbons with transition metals. Neutron diffraction evidence for an activated carbon-hydrogen bond in an electron-deficient tantalum- neopentylidene complex, J. Am. Chem. Soc. 101 (1979) 1593-1595. |
| [11] | D.H. Berry, J.H. Chey, H.S. Zipin, P.J. Carroll, Reactivity of molybdenum and tungsten disilene complexes, Polyhedron 10 (1991) 1189-1201. |
| [12] | D.H. Berry, J.H. Chey, H.S. Zipin, P.J. Carroll, Disilene complexes of molybdenum and tungsten, J. Am. Chem. Soc. 112 (1990) 452-453. |
| [13] | K.R. Grünwald, G. Saischek, M. Volpe, N.C. Mösch-Zanetti, Mechanistic insight into olefin epoxidation catalyzed by rhenium(v) oxo complexes that contain pyridazine- based ligands, Inorg. Chem. 50 (2011) 7162-7171. |
| [14] | A. Schröckeneder, P. Traar, G. Raber, et al., Oxorhenium(V) complexes with ketiminato ligands: coordination chemistry and epoxidation of cyclooctene, Inorg. Chem. 48 (2009) 11608-11614. |
| [15] | A. Jimtaisong, R.L. Luck, Synthesis and catalytic epoxidation activity with TBHP and H2O2 of dioxo-, oxoperoxo-, and oxodiperoxomolybdenum(VI) and tungsten( VI) compounds containing monodentate or bidentatephosphine oxide ligands: crystal structures of WCl2(O)2(OPMePh2)2, WCl2(O)(O2)(OPMePh2)2, MoCl2(O)2dppmO2 C4H10O, WCl2(O)2dppmO2, Mo(O)(O2)2dppmO2, and W(O)(O2)2dppmO2, Inorg. Chem. 45 (2006) 10391-10402. |
| [16] | R. Zhang, W.Y. Yu, K.Y. Wong, C.M. Che, Highly efficient asymmetric epoxidation of alkenes with a D4-symmetric chiral dichlororuthenium(IV) porphyrincatalyst, J. Org. Chem. 66 (2001) 8145-8153. |
| [17] | S. Bhor, M.K. Tse, M. Klawonn, C. Dobler, M. Beller, Ruthenium-catalyzed asymmetric alkene epoxidation with tert-butyl hydroperoxide as oxidant, Adv. Synth. Catal. 346 (2004) 263-267. |
| [18] | R.I. Kureshy, N.H. Khan, S.H.R. Abdi, Enantioselective catalytic epoxidation of styrenes by iodosylbenzene using chiral ruthenium(Ⅱ) schiff base complexes, J. Mol. Catal. A: Chem. 96 (1995) 117-122. |
| [19] | R.I. Kureshy, N.H. Khan, S.H.R. Abdi, A.K. Bhatt, Asymmetric catalytic epoxidation of styrene by dissymmetric Mn(iii) and Ru(iii) chiral schiff base complexes synthesis and physicochemical studies, J. Mol. Catal. A: Chem. 110 (1996) 33-40. |
| [20] | T. Takeda, R. Irie, Y. Shinoda, T. Katsuki, Ru-salen catalyzed asymmetric epoxidation: photoactivation of catalytic activity, Synlett 7 (1999) 1157-1159. |
| [21] | C. Stoe, X-STEP32 Version 1.07b: Crystallographic package, GmbH, Darmstadt, Germany, 2000. |
| [22] | A. Altomare, G. Cascarano, C. Giacovazzo, et al., A program for automatic solution of crystal structures by direct methods optimized for powder data, J. Appl. Cryst. 27 (1994) 435-436. |
| [23] | G.M. Sheldrick, University of Gottingen, Germany, 2008. |
| [24] | O.B. Deacon, R.J. Phillips, Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination, Coord. Chem. Rev. 33 (1980) 227-250. |
| [25] | C. Yin, G.C. Huang, C. KuoKuo, et al., Extended metal-atom chains with an inert second row transition metal: [Ru5(μ5-tpda)4X2] (tpda2- = tripyridyldiamidotripyridyldiamidodianion, X = Cl and NCS), J. Am. Chem. Soc. 130 (2008) 10090-10092. |
| [26] | J. Hine, Carbon dichloride as an intermediate in the basic hydrolysis of chloroform. A mechanism for substitution reactions at a saturated carbon atom, J. Am. Chem. Soc. 72 (1950) 2438-2445. |