It has been recognized that the physical and chemical properties of inorganic nanomaterials such as magnetic,catalytic,optical, electronic properties,etc.are very sensitive to their size,dimension, and morphology [1, 2, 3, 4]. Therefore,systematic manipulation of the size and shape of inorganic compounds and investigation on the size and shape-dependent properties are being pursued actively.
As a wide band gap semiconductor (3.5 eV),BiOCl recently proved to be a promising semiconductor photocatalyst for treatment of organic contaminates and waste water due to its high photocatalytic activity and high chemical stability [5, 6, 7]. A relatively positive conduction band and the unique layered structures along thec-axis enable the easier electron transfer from the excited dye to BiOCl and effective separation of the photo-induced electron-hole pairs,thus making BiOCl an effective dye sensitization photocatalyst under visible light [8, 9, 10]. Over the past decade,many efforts have been devoted to synthesizing different BiOCl nanostructures including nanowires [11],nanobelts [12],nanosheets [13],nanoparticles [14], nanoflowers [15, 16],and hierarchical nanostructures [17, 18, 19, 20, 21, 22, 23] and to investigate their properties. Zhang’s group synthesized BiOCl singlecrystalline nanosheets and studied their facet-dependent photoreactivity [10]. Porous BiOCl micro-flowers composed of nanosheets prepared in glycerol exhibit good activity for indirect photosensitization degradation of RhB under visible light [16]. Although many different BiOCl nanostructures have been synthesized,surfactants and toxic organic solvents are usually used to modulate the morphology and size of the products. In contrast,an additive-free and template-free method offers more convenient and green ways to fabricate nanostructures. Thus,it is still of great importance to develop simple and environment friendly methods to realize tunable synthesis of BiOCl nanomaterials and investigate their size and shape-dependent properties. Herein,we present a simple,additivefree,and environmentally benign method for synthesizing several different BiOCl nanostructures through changing the volume ratio of PEG and H2O in the solvent. The photocatalytic activity of these nanostructures towards RhB was investigated. 2. Experimental 2.1. Synthesis
BiOCl nanostructures were prepared by a solvothermal process using a mixture of polyethylene glycol 400 (PEG 400) and distilled water as solvent. Typically,0.1000 g of Bi(NO3)3·5H2O and 0.1000 g of NaCl were dissolved into the solvent and then sealed into a Teflon-lined autoclave heated at 140℃ for 6 h. The products were collected and washed by centrifugation. The detailed experimental parameters and results are shown in Table 1.
| Table 1 Experimental conditions and results of samples. |
The phase and purity of the samples were characterized by Xray diffraction (XRD) on a Bruker D8 ADVANCE diffractometer. The morphology of the products was characterized by a field-emission scanning electron microscope (FEI,NOVA Nano SEM 230) and a high-resolution transmission electron microscope (HRTEM,FEI, Tecnai G2 F20). UV-vis diffuse reflectance spectra (DRS) were recorded on a UV-vis spectrometer (Perkin-Elmer,Lamda-35) using BaSO4 as a reference. The Brunauer-Emmett-Teller (BET) specific surface area of the powders was analyzed by nitrogen adsorption using a Micromeritics 3Flex nitrogen adsorption apparatus (USA). 2.3. Characterization of photocatalytic activity
The degradation reactions were conducted in an XPA-7 type photochemical reactor (Xujiang Machine Factory,Nanjing,China). Photocatalytic experiments were conducted as follows. 30 mg of different samples were immersed in Rhodamine B (RhB) solution (3.0×10-5 mol/L,60 mL) and then magnetically stirred in the dark for 30 min,allowing it to reach adsorption equilibrium and uniform dispersity. The solution was then exposed to visible light irradiation from a 500 W xenon lamp with a 420 nm cutoff filter at room temperature. At given time intervals,10 mL aliquots were sampled and centrifuged immediately to remove the photocatalyst particles. Then,the concentration of the dye solution was analyzed with a UV-vis spectrophotometer (752,Shanghai Hengping) by monitoring the absorption peak 553 nm. 3. Results and discussion
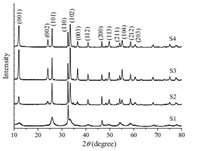
The phase purity and crystallinity of the as-synthesized products was characterized by powder XRD analysis. The XRD patterns of samples S1-S4 are shown in Fig. 1. All the diffraction peaks can be indexed to a tetragonal phase BiOCl (Bismoclite, a= 3.891 Å ,c= 7.369 Å ,JCPDS no. 06-0249) without the presence of other diffraction peaks,indicating the high purity of the products. The strong and narrow sharp diffraction peaks indicated that the as-synthesized products were well crystallized. Most notable in the XRD patterns are the increase in intensity of the {0 0 1} diffraction peak for the BiOCl microplates (S3) and nanoplates (S4),which implies that the basal planes of the nanosheets should be mainly dominated by their {0 0 1} facet,and therefore their {0 0 1} plane tended to be preferentially oriented parallel to the surface of the substrate in the XRD experiment.
|
Download:
|
| Fig. 1. XRD patterns of the BiOCl nanostructures. | |
Fig. 2a presents a low magnification SEM image of the products S1,showing that they are microspheres with a diameter ofca.1- 1.5mm. Fig. 2b is a higher magnification SEM image of the products,indicating clearly that the microspheres were composed of nanosheets ofca.17 nm in thickness. SEM images of products S2 (Fig. 2c and d) show bigger flower-like morphology. The microflowers were of ca. 9-16mm in diameter. The typical low magnification SEM image of product S3 in Fig. 2e clearly shows that the products were microplates of severalmm in lateral size. A higher magnification TEM image (Fig. 2f) further confirms the plate-like morphology of the products. The selected area electron diffraction (SAED) pattern corresponding to a single microplate (inset of Fig. 2f) shows well-defined sharp ED spots,which can be indexed to the (2 0 0),(1 1 0),and their equivalent planes. It is suggested that the as-prepared BiOCl nanoplates are single crystals. The SAED pattern of the nanoplate was projected from the [0 0 1] zone axis of a single crystal BiOCl plate. Fig. 2g and h present the typical SEM and TEM images of product S4,where nanoplate morphology with lateral size ofca.160-710 nm can be seen clearly. Some nanoplates exhibit square morphology. The SAED pattern corresponding to a single nanoplate (inset of Fig. 2h) was similar to that of S3 (inset of Fig. 2f),indicating that the bottom and top surfaces of both the two plates can be identified as {0 0 1} planes. These results agreed with the intensity increase of {0 0 1} diffraction peak in the XRD patterns.

|
Download:
|
| Fig. 2. SEM and TEM images of the BiOCl nanostructures: (a,b) S1; (c,d) S2; (e,f) S3; (g,h) S4. | |
As reported previously,using polyols such as ethylene glycol, glycerol,diethylene glycol,and triethylene glycol as solvent can facilitate the formation of hierarchical nanostructures due to the soft template effect of the solvent molecules [14, 15, 16, 17, 18]. Herein, PEG400 is a low molecule condensation polymer of ethylene oxide and water,which can play a similar role in the formation of hierarchical nanostructures. In addition,the viscosity of the solvent can affect the ion transfer rate and diffusion rate of the crystal nuclei and therefore play important roles in determining the morphology and size of final nanostructures. When pure PEG400 was used as the solvent,microspheres of compactly packed nanoplates were formed probably due to the soft template effect of the solvent molecules. When a part of the PEG400 solvent was replaced by water,the viscosity of the solvent decreases since the viscosity of water is much lower than that of PEG. Low viscosity may induce faster ion transfer rate and thus make the diffusion of BiOCl nuclei easier,so larger and loosely packed microspheres were obtained. Then,as the proportion of water in the solvent increased further,the template effect of PEG decreased and only plates with exposed {0 0 1} facets were formed due to the anisotropic growth habit of BiOCl [18] crystal and preferential adsorption of PEG molecules on {0 0 1} facets [16, 24, 25]. Thus,the morphology of the products can be modulated easily by adjusting the volume ratio of PEG400 and H2O in the solvent.
Fig. 3 shows the UV-vis diffuse reflection absorption spectra of different BiOCl nanostructures. The calculated band gap energies of S1,S2,S3 and S4 are calculated to be ca. 3.31,3.23,3.27,and 3.34 eV,respectively,which is slightly different from the theoretical value (3.46 eV) [13]. It can be clearly observed that the BiOCl microspheres ofca.1-1.5mm in diameter (S1) exhibit stronger absorption intensity in both the ultraviolet and visible light ranges. As is well known,specific surface area is another critical factor influencing the photocatalytic activity of photocatalysts. Thus,the Brunauer-Emmett-Teller (BET) specific surface areas of the four different BiOCl nanostructures were investigated by using nitrogen adsorption-desorption isotherms,and the corresponding BET surface areas were shown in Table 2. The BET surface areas of the samples S1,S2,S3,and S4 were 17.85,7.08, 6.36,and 7.52m2 /g,respectively. The BET value of the BiOCl microspheres of ca. 1-1.5mm in diameter (S1) was much larger than that of the other three samples. The enhanced light absorption capacity in addition to the relatively large BET specific surface areas may make the sample S1 a promising photocatalyst.

|
Download:
|
| Fig. 3. UV-vis diffuse reflectance spectra (DRS) of the BiOCl nanostructures | |
| Table 2 Brunauer-Emmett-Teller (BET) specific surface areas of the as-obtained BiOCl samples. |
The visible light photocatalytic activities of different BiOCl products were investigated by monitoring the degradation of RhB. Fig. 4a shows the temporal UV-vis spectra of the RhB solutions irradiated by visible light for different times in the presence of S1. The intensity of the major absorption peaks in the visible and UV regions decreased continuously along with irradiation time and completely disappeared after 40 min,indicating that the BiOCl sample showed good photocatalytic activity towards RhB. To further investigate the degradation efficiency and compare the photocatalytic activities of different BiOCl samples,the real-time degradation curves of RhB as a function of irradiation time is presented in Fig. 4b. For comparison,a blank experiment without any catalysts and two controlled experiments using commercial P25 (TiO2Degussa) and commercial BiOCl as the catalyst were also conducted,respectively. It was observed that RhB concentration remained almost unchanged in the absence of photocatalyst (blank experiment). For the controlled experiments,RhB degradation was relatively slow. The concentration decreased to about 23% of the initial for commercial P25 and about 10% of initial for commercial BiOCl within 60 min. In the presence of BiOCl nanostructures under the same experimental conditions,the degradation rate of RhB was increased. The BiOCl microspheres (S1) exhibited superior photocatalytic activity,and about 99.0% of RhB was degraded within 40 min. The photocatalytic activity of the nanoplates (S4) and bigger microflowers (S2) was similar,and 97.7% and 96.1% of RhB was degraded within 60 min,respectively. In summary,the photocatalytic reactivity of the four BiOCl samples follows the series of microspheres (S1)>nanoplates (S4)>bigger microflowers (S2)>microplates (S3). As a layered compound,the unique layered structure of BiOCl along the [0 0 1] direction can promote the effective separation of the photo-induced electron- hole pairs and enhance the photocatalytic activity of BiOCl. Thus, all four samples exhibit excellent visible-light-driven photocatalytic activity towards RhB. It has been recognized that the photocatalytic properties of nanomaterials are very sensitive to their size,dimension,morphology,and composition [4, 26, 27]. Therefore,the four samples with different morphology and size exhibit slightly different photocatalytic activity towards RhB. It has been proven that RhB degradation on the wide gap BiOCl nanostructures under visible light irradiation mainly proceeds along a photosensitization pathway. For sensitization photocatalysis,direct interaction between the dye molecules and the surface of the catalyst is necessary to achieve an efficient charge between the excited state of dye and BiOCl. Therefore,large surface area can promote the adsorption of RhB on the catalyst and facilitate subsequent photodegradation reactions [10, 13, 16, 18]. Among the four BiOCl samples measured,the BET surface area of the BiOCl microspheres ofca.1-1.5mm in diameter (S1) was much larger than the other three samples,therefore the microspheres show better performance in the photodegradation of the dye.

|
Download:
|
| Fig. 4. Photocatalytic degradation of RhB over the BiOCl nanostructures under visible-light illumination. (a) UV-vis spectral changes of the RhB aqueous solutions as a function of irradiation time in the presence of S1; (b) the change in RhB concentration based on the absorbance ratioC/C0as a function of irradiation time. HereC0andCare the absorbances from the initial solution and after visible-light irradiation,respectively. (Blank: photolysis of RhB solution only.). | |
In summary,BiOCl microspheres,microflowers,microplates, and nanoplates have been synthesized in a simple PEG/H2O system without the assistance of any additives. The volume ratio of PEG and H2O in the solvent significantly influenced the morphology and photocatalytic activity of the resulting BiOCl samples. Soft template effect and preferential adsorption of PEG molecules may play key roles in adjusting the morphology of the products. Among the four BiOCl samples,the microspheres exhibited superior photocatalytic activity,probably due to their larger surface area.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21001081,21043004) and the Education Commission of Tianjin (No. 20110510).
| [1] | C. Burda, X. Chen, R. Narayanan, M.A. El-Sayed, Chemistry and properties of nanocrystals of different shapes, Chem. Rev. 105 (2005) 1025-1102. |
| [2] | J.H. Lee, Gas sensors using hierarchical and hollow oxide nanostructures: overview, Sens. Actuators B 140 (2009) 319-336. |
| [3] | L. Peng, L. Hu, X. Fang, Energy harvesting for nanostructured self-powered photodetectors, Adv. Funct. Mater. 24 (2014) 2591-2610. |
| [4] | S. Han, L. Hu, N. Gao, A.A. Al-Ghamdi, X. Fang, Efficient self-assembly synthesis of uniform CdS spherical nanoparticles-Au nanoparticles hybrids with enhanced photoactivity, Adv. Funct. Mater. 24 (2014) 3725-3733. |
| [5] | X.P. Lin, T. Huang, F.Q. Huang, W.D. Wang, J.L. Shi, Photocatalytic activity of a Bibased oxychloride Bi3O4Cl, J. Phys. Chem. B 110 (2006) 24629-24634. |
| [6] | J. Henle, P. Simon, A. Frenzel, S. Scholz, S. Kaskel, Nanosized BiOX(X = Cl, Br, I) particles synthesized in reverse microemulsions, Chem. Mater. 19 (2007) 366-373. |
| [7] | M.A. Gondala, X.F. Chang, Z.H. Yamani, UV-light induced photocatalytic decolorization of Rhodamine 6G molecules over BiOCl from aqueous solution, Chem. Eng. J. 165 (2010) 250-257. |
| [8] | K.L. Zhang, C.M. Liu, F.Q. Huang, C. Zheng, W.D. Wang, Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst original, Appl. Catal. B 68 (2006) 125-129. |
| [9] | L.Q. Ye, L. Zan, L.H. Tian, T.Y. Peng, J.J. Zhang, The {0 0 1} facets-dependent high photoactivity of BiOCl nanosheets, Chem. Commun. 47 (2011) 6951-6953. |
| [10] | J. Jiang, K. Zhao, X.Y. Xiao, L.Z. Zhang, Synthesis and facet-dependent photoreactivity of BiOCl single-crystalline nanosheets, J. Am. Chem. Soc. 134 (2012) 4473-4476. |
| [11] | S. Wu, C. Wang, Y. Cui, et al., Synthesis and photocatalytic properties of BiOCl nanowire arrays, Mater. Lett. 64 (2010) 115-118. |
| [12] | H. Deng, J. Wang, Q. Peng, X. Wang, Y. Li, Controlled hydrothermal synthesis of bismuth oxyhalide nanobelts and nanotubes, Chem. Eur. J. 11 (2005) 6519-6524. |
| [13] | J. Xiong, G. Cheng, G. Li, F. Qin, R. Chen, Well-crystallized square-like 2D BiOCl nanoplates: mannitol-assisted hydrothermal synthesis and improved visiblelight- driven photocatalytic performance, RSC Adv. 1 (2011) 1542-1553. |
| [14] | B. Pare, B. Sarwan, S.B. Jonnalagadda, The characteristics and photocatalytic activities of BiOCl as highly efficient photocatalyst, J. Mol. Struct. 1007 (2012) 196-202. |
| [15] | J. Song, C. Mao, H. Niu, Y. Shen, S. Zhang, Hierarchical structured bismuth oxychlorides: self-assembly from nanoplates to nanoflowers via a solvothermal route and their photocatalytic properties, CrystEngCommun 12 (2010) 3875- 3881. |
| [16] | D.H. Wang, G.Q. Gao, Y.W. Zhang, et al., Nanosheet-constructed porous BiOCl with dominant {0 0 1} facets for superior photosensitized degradation, Nanoscale 4 (2012) 7780-7785. |
| [17] | X. Zhang, Z. Ai, F. Jia, L. Zhang, Generalized one-pot synthesis, characterization, and photocatalytic activity of hierarchical BiOX (X = Cl, Br, I) nanoplate microspheres, J. Phys. Chem. C 112 (2008) 747-753. |
| [18] | J. Xiong, G. Cheng, F. Qin, et al., Tunable BiOCl hierarchical nanostructures for high-efficient photocatalysis under visible light irradiation, Chem. Eng. J. 220 (2013) 228-236. |
| [19] | Z.K. Cui, L.W. Mi, D.W. Zeng, Oriented attachment growth of BiOCl nanosheets with exposed {1 1 0} facets and photocatalytic activity of the hierarchical nanostructures, J. Alloys Compd. 549 (2013) 70-76. |
| [20] | L.P. Zhu, G.H. Liao, N.C. Bing, et al., Self-assembled 3D BiOCl hierarchitectures: tunable synthesis and characterization, CrystEngComm 12 (2010) 3791-3796. |
| [21] | Y. Lei, G. Wang, S. Song, W. Fan, H. Zhang, Synthesis, characterization and assembly of BiOCl nanostructure and their photocatalytic properties, CrystEng- Comm 11 (2009) 1857-1862. |
| [22] | J. Xiong, Z. Jiao, G. Lu, et al., Facile and rapid oxidation fabrication of BiOCl hierarchical nanostructures with enhanced photocatalytic properties, Chem. Eur. J. 19 (2013) 9472-9475. |
| [23] | J. Liu, J. Hu, L. Ruan, Y. Wu, Facile and environment friendly synthesis of hierarchical BiOCl flowery microspheres with remarkable photocatalytic properties, Chin. Sci. Bull. 59 (2014) 802-809. |
| [24] | W.Q. Fang, J.Z. Zhou, J. Liu, et al., Hierarchical structures of single-crystalline anatase TiO2 nanosheets dominated with {0 0 1} facets, Chem. Eur. J. 17 (2011) 1423-1427. |
| [25] | Z.H. Zhang, S.H. Liu, S.Y. Chow, M.Y. Han, Modulation of the morphology of ZnO nanostructures via aminolytic reaction: from nanorods to nanosquamas, Langmuir 22 (2006) 6335-6340. |
| [26] | H. Wang, B. Wang, S. Ma, Synthesis of visible-light-driven TiO2 yolk-shell spheres with {0 0 1} facets dominated mesoporous shells, Chin. Chem. Lett. 24 (2013) 260-263. |
| [27] | Z.P. Li, Y.Q. Wen, J.P. Shang, et al., Magnetically recoverable Cu2O/Fe3O4 composite photocatalysts: fabrication and photocatalytic activity, Chin. Chem. Lett. 25 (2014) 287-291. |